Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Exosomes are microvesicles that can be secreted by various cells and carry a variety of contents; thus, they play multiple biological functions. For instance, the tumor-derived exosomes (TEXs) have been proven to have the effect of immunostimulatory in addition to immunosuppression, making TEXs attractive in clinical immunotherapy and targeted therapy for cancer patients. In addition, TEXs as biomarkers have important clinical diagnostic and prognostic value.
- tumor-derived exosomes
- biomarkers
- immunomodulation
- abscopal effect
- radiotherapy
1. Exosomes
Exosomes are a kind of extracellular vesicles that are formed inside the cells and secreted from the cells through exocytosis. Exosomes differ from microvesicles larger than 100 nm in diameter, with an average diameter of 30–100 nm. The biogenesis of exosomes starts from the invagination of the plasma membrane to form endosomes controlled by the endosomal system. Then, during the process with the participation of Golgi bodies, exosomes are formed from endosomes in the early phase to multivesicular bodies in the late phase, and the membrane of multivesicular bodies could form a lumen where intraluminal vesicles are contained. Then the multivesicular bodies could be degraded by lysosomes or fused with plasma membrane, and the intraluminal vesicles could be released outside the cell through exocytosis to form exosomes.
The process above is regulated by Rab GTPases and the endosomal-sorting complex required for transport (ESCRT) system. The Rab GTPases in this study included Rab14, Rab22, Rab27 and Rab37, which were responsible for the formation of intraluminal vesicles, the fusion of the multivesicular bodies with the plasma membrane and the degradation of the multivesicular bodies by lysosomes. The ESCRT included ESCRT-0, ESCRT-I, ESCRT-II and ESCRT-III. ESCRT-0 was responsible for assembling molecules and internalizing the ubiquitinated proteins. ESCRT-1 and ESCRT-II were responsible for the invagination process and promoting the deubiquitination of those ubiquitinated proteins. ESCRT-III was responsible for the separation and disassembling of intraluminal vesicles [9].
The exosomes only can be observed under the electron microscopy. Although there are many kinds of exosomes which are composed of different molecules that they transfer, the morphology of the exosomes is similar. Morphologically, the exosomes in our study were round in shape, with a phospholipid bilayer membrane and aqueous core containing proteins and nucleic acids. The molecules contained in exosomes were dependent on their parental cells, their pathophysiological conditions and environmental stimulations. Generally, as shown in Figure 1, the content of exosomes includes major histocompatibility complex class I and II (MHC I and II), heat shock proteins (HSP), tetraspanins (such as CD9, CD63 and CD81), integrins, intercellular adhesion molecule 1 (ICAM-1), lysosomal-associated membrane glycoprotein 1/2 (LAMP1/2), nucleic acids including miRNA, lncRNAs, mRNA and DNA, cytoplasmic proteins and other membrane-bounded proteins [9]. In addition to these proteins and nucleic acids, there were also cholesterol, sphingomyelin, phosphatidylethanolamine and phosphatidylcholine on the cell membranes of the exosomes in our study [10].
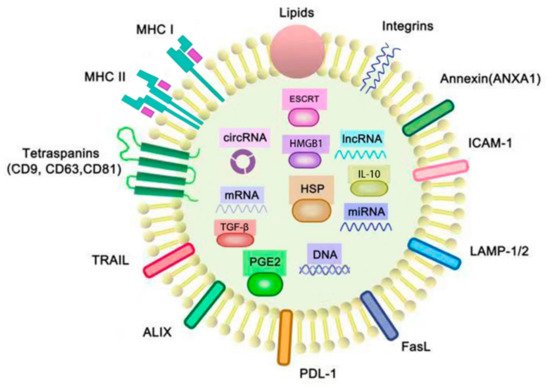
Figure 1. Structure and cargoes of exosomes. The membranes of exosomes consist of phospholipid bilayers, proteins and lipids, and there are many molecules contained in the aqueous core of exosomes. These molecules include the cytoplasmic proteins: the endosomal-sorting complex required for transport (ESCRT), prostaglandin E2 (PGE2), heat shock protein (HSP), high-mobility group box 1 (HMGB1), TGF-β and IL-10; the membrane proteins: integrins, intercellular adhesion molecule 1 (ICAM-1), lysosomal-associated membrane glycoprotein 1/2 (LAMP-1/2), FasL, PDL-1, ALIX, TRAIL, tetraspanins (CD9, CD63 and CD81), major histocompatibility complexes I and II (MHC I, II) andANXA1; and the nucleic acids: miRNA, mRNA, circRNA and lncRNA. FasL, PD-L1, ALIX, TRAIL, PGE2, IL-10, TGF-β and tumor-associated antigens (TAAs) are typically found in TEXs for immunosuppression, whereas HSPs like HSP 70 and HSP 80, MHC molecules and tetraspanins are found in TEXs which are related with the functions of immunostimulatory. ANXA1 is a kind of annexin that not only plays a key role in cell–cell adhesion, but also inhibits tumor-induced inflammatory responses.
2. Tumor-Derived Exosomes (TEXs)
TEXs contain some extra immune suppression-associated molecules, such as tumor-associated antigens, FasL, PD-L1, ALIX, TRAIL, IL-10, TGF-β and prostaglandin E2 (PGE2) [5], which are immunosuppressive molecules and were shown to induce the inhibition of dendritic cells, the differentiation apoptosis of T cells, the differentiation of Tregs and the induction of myeloid-suppressive cells. On the other hand, TEXs are composed of immune stimulatory molecules such as co-stimulatory molecules and MHC molecules. Therefore, TEXs possess dual signaling abilities including immunostimulatory and immunosuppression [11].
The main functions of TEXs are determined by the role they play in the tumor microenvironment. Therefore, because of the abilities of intercellular communication and immune regulation, TEXs play a major role in cancer progression by the bioactive molecules interacting with the recipient cells and changing their functions. Research has demonstrated that TEXs are the carriers of oncogenetic signals and oncogenes to promote the process of neoplastic transformation. In general, TEXs were proven to be related to tumor progression, angiogenesis, drug resistance and immune regulation. Specifically, TEXs promote tumor progression through the upregulation of the tumor cell proliferation and tumor growth, the enhancement of tumor invasion and tumor metastasis, the suppression of anti-tumor immune responses and the promotion of the horizontal transfer of oncogenic mutations [12]. Epidermal growth factor (EGF-EGFR) and phosphatase and tensin homolog deleted on chromosome 10 (PI3K/AKT—PTEN) in TEXs are mostly involved in metastasis [13], and drug resistance including the removal of toxic drugs [14] and exchange of drug transporters [15]. Transportation of multidrug resistance (MDR)-associated miRNAs by TEXs have been associated with the elevation of drug resistance [13]. The angiogenesis is also involved in this process of TEX-based regulation through the following signaling pathways. The vascular endothelial growth factor (VEGF-VEGFR), TGF-β, and fibroblast growth factor (FGF-FGFR) in TEXs are mostly involved in angiogenesis [16].
2.3. Isolation of TEXs from Cancer Patients
Research has shown that most of the tumor-derived exosomes are isolated from the supernatants of cultured tumor cells [5]. Various methods used to isolate TEXs from the body fluid have been developed, which include complicated procedures of purification and recovery. For example, TEXs have been isolated by ultracentrifugation, filtration, immunoaffinity-based isolation and microfluidics techniques [17]. Ultracentrifugation is the most commonly used method to separate the exosomes, which based on the size and buoyant density. However, ultracentrifugation is time consuming, and has low recovery and low specificity. Ultracentrifugation also requires expensive equipment and specific technicians.
The density-gradient separation is another conventional method with a better purity and recovery rate for isolating exosomes. This separation method is based on the isopycnic point mechanism [10]. Although this method can achieve the higher recovery, purity and specificity, the disadvantages are almost the same as ultracentrifugation, such as being time consuming and requiring expensive equipment.
Immunoaffinity-based isolation is a method that depends on specific proteins on the membrane of exosomes. Antibody-conjugated beads are used in this method to recognize the specific antigens on the exosomes membrane and then capture the TEXs. This technique has more advantages: the reduction of cell-debris and protein aggregates in co-purification, better isolation of exosomes subgroups based on the specific antigens on the exosomes membranes, increased recovery rate and the ability to process multiple samples simultaneously [18,19].
There have also been other techniques used for isolation of exosomes, such as using the force of inertia in microfluidic channels to separate the exosomes from the other substances [10] and the lectin-induced aggregation of exosomes, which has a lower cost and easy operation [20]. Although these exosome isolation methods are practical for using in clinical practice, the problems in the isolation of TEXs still need to be properly resolved, including choosing the proper donor cells, the method of purification and amplification, cost and time [21]. Thus, techniques need to be further developed in order to isolate and handle TEXs properly and quickly in clinical settings.
This entry is adapted from the peer-reviewed paper 10.3390/life11050381
This entry is offline, you can click here to edit this entry!
