The aquathermolysis process is widely considered to be one of the most promising approaches of in-situ upgrading of heavy oil.
- heavy oil
- in-situ upgrading
- transition metals
- aquathermolysis
- catalyst
1. Introduction
Heavy oil stands for one-third of global hydrocarbon resources. Only in Russia, the reserves of heavy oil are estimated at 6–7 billion tons [1]. The depletion of traditional hydrocarbon resources and the increasing demand of energy makes the enhancement of heavy oil recovery methods relevant and crucial. However, the high viscosity, significant content of heavy molecular mass fractions and high heteroatom-containing compounds with complex structures and compositions make the production, transportation and refinery of heavy oil very difficult and challenging. Currently, steam-assisted gravity drainage (SAGD), steam and hot water flooding, cyclic steam stimulation (CSS), and in-situ combustion (ISC) heavy oil recovery techniques are widely applied and very attractive due to their physical consequences. However, the feasibility of these techniques can be improved by accelerating the overall chemical reactions occurring between the steam, heavy oil and rock minerals, which were termed by Hyne et al. as “Aquathermolysis” [2]. The authors proposed the catalytic role of metal ions in the aquathermolysis process. Since then, substantial advances in catalysis have made the catalytic aquathermolysis a distinct process, and catalytic steam-based heavy oil recovery techniques has been investigated as a promising technology to recover heavy oil resources [3][4][5][6][7][8][9][10][11][12][13][14][15][16]. More importantly, this process occurs in the cheapest high-pressure reactor of all, petroleum formations [3][4][5][6]. Thus, the catalytic aquathermolysis process provides not only feasible enhancement of heavy oil recovery, but also contributes to its further transportation and refinery by changing the quality of difficult-to refine feedstock in-place. This paper sheds new light on the process of upstream heavy oil aquathermolysis in the presence of transition metal-based catalysts.
2. Catalytic Aquathermolysis
An increasing number of studies have found that steam flooding of heavy oil and oil-saturated rocks results in the destruction of the weakest carbon–heteroatom bonds with detachment of peripheral fragments from resins and asphaltenes and formation of lighter hydrocarbons [1][2][3][4][5][6][7][8][9][10][11]. The destruction of asphaltenes under the steam treatment occurs primarily through the weak aliphatic sulfide bonds (Figure 1) that are destabilized by electron-withdrawing functional groups. Such a process initiates already at 120 °C [13]. The destruction products of resins and asphaltenes joins other fractions, which determines reduction in heavy oil viscosity and increase in heavy oil recovery [3][4][7]. The schematics of the main chemical reactions that describe the catalytic aquathermolysis process are illustrated in Figure 1. The attempt was done to interrelate the products of one chemical reaction to the feed of another one. According to the proposed mechanism, we believe that the weak C-S bonds in heavy oil are very sensitive and under the steam exposure act like a trigger to initiate the overall chemical reactions, which make up the aquathermolysis process. Moreover, the transition metal species can accelerate certain chemical reactions as illustrated in Figure 1.
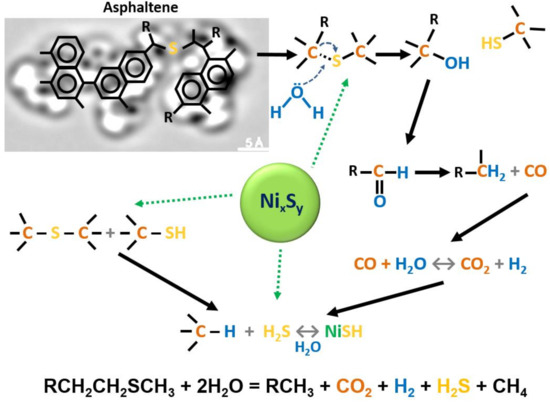
Figure 1. The schematics of main chemical reactions during the aquathermolysis process in the presence of nickel sulfide catalyst.
Lin et al. reported that catalyst particles are retained on the surface of silicate particles and accelerate the chemical rupture of carbon–heteroatom bonds [14]. They draw our attention to the significant amount of H2S generation after catalytic aquathermolysis of tetra-hydro-thiophene, as a model compound [14]. The authors consider C-S bond cleavage and ring opening reactions, which lead to the formation of low-molecular alkanes and alkenes (Figure 2).

Figure 2. Reaction pathways for tetrahydrothiophene aquathermolysis.
Decarbonylation of aldehydes and other organic compounds leads to the formation of CO, hydrocarbons and other related compounds. The high-temperature water plays a key role during the aquathermolysis of tetra-hydro-thiophene and is directly involved in the main reactions such as hydrolysis and water–gas shift (WGS). These simple reactions will eventually lead to the transfer of hydrogen from water into gaseous or liquid phase products. In addition, the kinetic model of H2S formation after aquathermolysis of tetra-hydro-thiophene was established, the activation energy of which was 54.55 kJ/mole [14].
In [10] the authors studied the group composition and elemental analysis after the thermolysis of asphaltenes, which were previously extracted from the heavy oil of the Usinskoye reservoir [10]. The temperature range of the carried out thermolysis process was 120–750 °C [10]. Gas Chromatography–Mass Spectrometry (GC-MS) was used to reveal the main thermolysis products such as alkylbenzenes (AB) and saturated aliphatic hydrocarbons (SAH), while aromatic structures obtained at higher temperatures were mainly separate aggregates [10]. The authors claim that the temperature of thermolysis is directly proportional with the ratio of AB/SAH + Alkenes and inversely proportional with phenanthrene/alkylbenzenes (PN/AB) and polycyclic aromatic hydrocarbons/alkylbenzenes (PAH/AB) ratios [10].
Wren Montgomery and his co-workers carried out an aquathermolysis process under various thermobaric conditions (up to 325 °C and 13.8 MPa) [16]. The authors affirm the generation of aliphatic hydrocarbons from polar resins and asphaltenes. The analysis of maturity parameters in the destruction products showed the lower temperatures than the feed material. According to the FTIR Spectroscopy results, the amount of methane gas gradually increased with temperature and pressure [16]. More importantly, FT-IR data justified the defunctionalization of polar components. The significant amount of methane and CO2 were detected in high temperature and pressure experiments, which is probably due to the cracking of C-C bonds.
This entry is adapted from the peer-reviewed paper 10.3390/pr9010127
References
- Yarboboev, T.; Sultanov, S.; Aminov, F.; Navotova, D. Non-traditional oils: Analysis of regional distribution and reserves of heavy oil and natural bitumen. Bull. Sci. Pract. 2020, 6, 226–234.
- Hyne, J.B. Aquathermolysis: A Synopsis of Work on the Chemical Reaction between Water (Steam) and Heavy Oil Sands during Simulated Steam Stimulation; Alberta Oil Sands Technology and Research Authority: Edmonton, AB, Canada, 1986; pp. 1–82.
- Muraza, O.; Galadima, A. Aquathermolysis of heavy oil: A review and perspective on catalyst development. Fuel 2015, 157, 219–231.
- Maity, S.K.; Ancheyta, J.; Marroquin, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuels 2010, 24, 2809–2816.
- Mullins, O.C.; Betancourt, S.S.; Cribbs, M.E.; Dubost, F.X.; Creek, J.L.; Andrews, A.B.; Venkataramanan, L. The colloidal structure of crude oil and the structure of oil reservoirs. Energy Fuels 2007, 21, 2785–2794.
- Antipenko, V.R.; Golubina, O.A.; Goncharov, I.V.; Nosova, S.V.; Ostroukhov, S.B. Specifics of the composition of monocyclic aromatic hydrocarbons in asphaltite from the Ivanovskoe deposit. Pet. Chem. 2007, 47, 154–161.
- Kayukova, G.P.; Gubaidullin, A.T.; Petrov, S.M.; Romanov, G.V.; Petrukhina, N.N.; Vakhin, A.V. Changes of asphaltenes’ structural phase characteristics in the process of conversion of heavy oil in the hydrothermal catalytic system. Energy Fuels 2016, 30, 773–783.
- Nasyrova, Z.R.; Kayukova, G.P.; Vakhin, A.V.; Djimasbe, R.; Chemodanov, A.E. Heavy oil hydrocarbons and kerogen destruction of carbonate-siliceous domanic shale rock in sub- and supercritical water. Processes 2020, 8, 800.
- Katritzky, A.R.; Barcock, R.A.; Siskin, M.; Olmstead, W.N. Aqueous high-temperature chemistry of carbo- and heterocycles. 23. reactions of pyridine analogs and benzopyrroles in supercritical water at 460 °C. Energy Fuels 1994, 8, 990–1001.
- Korneev, D.S.; Melenevskii, V.N.; Pevneva, G.S.; Golovko, A.K. Group composition of hydrocarbons and hetero compounds in stepwise-thermolysis products of asphaltenes from usa oil. Pet. Chem. 2018, 58, 179–185.
- Hyne, J.B.; Greidanus, J.W.; Tyrer, J.D.; Verona, D.; Clark, P.D.; Clarke, R.A.; Koo, J.H.F. «The Future of Heavy Crude and Tar Sands» Aquathermolysis of heavy oils. In Proceedings of the 2nd International Conference, Caracas, Venezuela, 7–17 February 1982; pp. 404–411.
- Schuler, B.; Meyer, G.; Peña, D.; Mullins, O.C.; Gross, L. Unraveling the molecular structures of asphaltenes by atomic force microscopy. J. Am. Chem. Soc. 2015, 137, 9870–9876.
- Korneev, D.S.; Pevneva, G.S.; Golovko, A.K. Thermal transformations of asphaltenes at a temperature of 120 °C. J. Sib. Fed. Univ. Chem. 2019, 12, 101–117.
- Lin, R.; Chen, K.; Miao, M.; Zhang, L.; Wang, X.; Jiang, Y.; Zhang, J.; Wang, Y.; Pan, H. Reaction mechanism of H2S generation during tetrahydrothiophene aquathermolysis reaction. Energy Fuels 2020, in press.
- Chemodanov, A.E.; Akhmadullin, R.R.; Sudakov, V.A.; Usmanov, S.A.; Khayrtdinov, R.K. Geochemical modeling with the use of vertical and horizontal relative concentrations of oil compounds for the heavy oil fields. Pet. Sci. Technol. 2018, 36, 1100–1106.
- Montgomery, W.; Court, R.W.; Rees, A.C.; Sephton, M.A. High temperature reactions of water with heavy oil and bitumen: Insights into aquathermolysis chemistry during steam-assisted recovery. Fuel 2013, 113, 426–434.
