Avian pathogenic Escherichia coli (APEC), an extra-intestinal pathogenic E. coli (ExPEC), causes diverse local and systemic infections in poultry, including chickens, turkeys, ducks, and many other avian species. APEC possesses or utilizes different virulence and pathogenesis factors or mechanisms to cause colibacillosis in poultry.
- APEC
- virulence
- pathogenesis
- zoonosis
- antibiotic resistance
- vaccines
- virulence inhibitors
- infections
1. Introduction
Avian pathogenic Escherichia coli (APEC), an extra-intestinal pathogenic E. coli (ExPEC), causes diverse local and systemic infections in poultry, including chickens, turkeys, ducks, and many other avian species [1]. The most common infections caused by APEC in chickens are perihepatitis, airsacculitis, pericarditis, egg peritonitis, salphingitis, coligranuloma, omphalitis, cellulitis, and osteomyelitis/arthritis; these are commonly referred as avian colibacillosis [2]. APEC also causes swollen head syndrome in chickens and osteomyelitis complex in turkeys [2]. Colibacillosis is one of the leading causes of mortality (up to 20%) and morbidity in poultry and also results in decreased meat (2% decline in live weight, 2.7% deterioration in feed conversion ratio) and egg production (up to 20%), decreased hatching rates, and increased condemnation of carcasses (up to 43%) at slaughter [1,3,4]. Furthermore, APEC is responsible for high mortality (up to 53.5%) in young chickens [4]. Taken together, along with the treatment expenses, APEC costs the poultry industry hundreds of millions of dollars in economic losses worldwide [5]. In the United States (US), it has been estimated that economic losses to the broiler industry can be as high as $40 million annually only due to carcass condemnations [6].
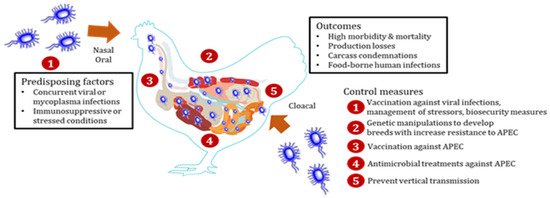
APEC can affect all species of poultry in all types of production systems [3]. APEC is also prevalent (9.52% to 36.73%) in all age groups of chickens [7]. Broiler chickens between the ages of 4 and 6 weeks are more susceptible [1], whereas layer chickens can be affected by APEC throughout the grow and lay periods, particularly around the peak egg production and late lay period [1]. In the US, it is estimated that at least 30% of commercial flocks are affected by APEC at any point of time [8]. Multiple APEC serotypes have been associated with colibacillosis cases in the field outbreaks; however, three serotypes (O78, O2, and O1) account for the majority (more than 80%) of the cases [1,5]. APEC leads to systemic infections in chickens either as a primary pathogen or secondary to viral [infectious bronchitis (IBV), Newcastle disease (NDV), avian influenza (AIV)] and Mycoplasma (Mycoplasma gallisepticum (MG) infections, immunosuppressive disease [(infectious bursal disease (IBD)], or environmental stresses (overcrowding, high level of dust and ammonia) by entering through oral and respiratory routes [1,5]. Interestingly, studies have shown that APEC can colonize the gastrointestinal and respiratory tracts of chickens without causing disease and only translocate to extra-intestinal sites in the presence of stressors (production-related stress, immunosuppression, and concurrent infections) as an opportunistic pathogen [2,9]. APEC invades the gastrointestinal and respiratory tracts through abraded tracheal and intestinal epithelium in the presence of stressors and reaches bloodstream and internal organs [1,2,3]. Chickens get infected through contaminated feed and water and can spread to other birds through the feco-oral or aerosol route [1,2,3]. Furthermore, APEC can be vertically transmitted from infected breeders via contaminated eggs [1,2,3]. An overview of APEC infection in chickens is shown in Scheme 1.
APEC utilizes different virulence and pathogenesis factors to cause disease in chickens, primarily adhesins, invasins, protectins, iron acquisition systems, and toxins [2]. These factors facilitate adhesion, invasion, evasion from the host immune responses, colonization, proliferation, and systemic dissemination of APEC, thereby allowing the establishment of infection in chickens [2]. In addition to these factors, several other bacterial factors including but not limited to secretion systems (type III and VI), quorum sensing (QS) system, transcriptional regulators, two-component systems, and metabolism-associated genes also contribute to APEC pathogenesis in chickens [10,11,12,13,14,15,16,17,18]. An in-depth understanding of these factors and their roles in APEC pathogenesis will help to develop new effective preventative and therapeutic treatments.
Recent studies suggest APEC (particularly isolates belonging to sequence types ST95 and ST131 or O1, O2, and O18 serogroups) as a potential foodborne zoonotic pathogen as well as a source or reservoir of extra-intestinal infections in humans [4,19,20,21]. Particularly, APEC shares genetic similarity with human ExPECs, uropathogenic E. coli (UPEC), and neonatal meningitis E. coli (NMEC), and possesses UPEC-and NMEC-defining virulence genes with the ability to cause urinary tract infections (UTI) and meningitis in mice and rat models [4,22]. Furthermore, the detection of APEC-specific ColV (colicin V) plasmids in human ExPEC isolates suggests a possible zoonotic transmission of APEC from poultry to humans [21]. Therefore, APEC is a pathogen of importance to the poultry industry and public health.
Antibiotics (tetracyclines, sulfonamides, and aminoglycosides) are frequently used to control colibacillosis in chickens [23]. However, increasing resistance of APEC to different classes of antibiotics, including medically important antibiotics (β-lactams, colistin, and carbapenems), suggests challenges ahead in using antibiotics to control APEC infections in chickens [24]. Furthermore, there is no effective vaccine available to protect chickens against APEC infections, which is mainly due to the diversity of APEC serotypes associated with colibacillosis cases in the field outbreaks [5]. Currently, only two vaccines (live-attenuated APEC O78 ΔaroA Poulvac® E. coli vaccine and inactivated Nobilis® E. coli vaccine containing F11 fimbrial and FT flagellar antigens) are commercially available for use in chickens [5,25]. These scenarios necessitate the development of new and alternative therapies to control APEC infections in chickens. Probiotics, bacteriophages, and different new therapies (innate immune stimulants, growth and QS inhibitors, and antimicrobial peptides) have shown promising efficacy in reducing APEC infections in chickens [26,27,28,29,30]; however, none of these have advanced into field applications to date.
2. Virulence and Pathogenesis Factors
APEC possesses or utilizes different virulence and pathogenesis factors or mechanisms to cause colibacillosis in poultry [2,10,11,12,13,14,15,16,17,18,31]. These factors include but are not limited to adhesins, invasins, protectins, iron acquisition systems, toxins, two-component systems, a quorum-sensing (QS) system, transcriptional regulators, secretion systems, and genes associated with metabolism [2,10,11,12,13,14,15,16,17,18,31]. These factors play various roles in APEC infections, including attachment to host cells, invasion of the host cells, survival inside the phagocytic (macrophages) cells, colonization of tissues, persistence in the bloodstream, proliferation/replication inside the cells, cell lysis and damage, sequestering metals from body fluids for growth, resistance to the serum bactericidal activity and oxidative and environmental stresses, motility, and biofilm formation [2,10,11,12,13,14,15,16,17,18,31]. Table 1 provides the list of virulence and pathogenesis factors defined or characterized in APEC to date along with their roles in APEC pathogenesis/infection.
| Virulence Factors | Genes/Proteins Involved | Role in Pathogenesis/Infection | Reference |
|---|---|---|---|
| Adhesins | fimH, fimC, papA, papC, papEF, papG I, papG II, papGIII, felA, sfa/sfaS, afaIBC, focGE, lpfA, lpf0141, lpf0154, flgE, crl, csg, bmaE, tsh, mat/ecpA, hra/hrlA/hek, iha, yqiG, kii | Adhesion, colonization, biofilm formation, motility, intracellular survival | [32,33,34,35,36,37,38,39,41] |
| yfc O | Adhesion, colonization, resistance to environmental stresses | [42] | |
| yad C | Adhesion, intracellular survival, motility | [43] | |
| aat A, aatB, upaB | Adhesion, colonization, biofilm formation | [44,45] | |
| fdtA, rluD, yjhB, ecpR, fdeC | Adhesion | [46] | |
| Invasins | ibeA, ibeB, tia, gimB | Invasion, resistance to oxidative stress, colonization, proliferation, biofilm formation | [35,47,48] |
| IbeR | Invasion, resistance to serum and environmental stresses, expression of virulence genes | [49] | |
| ych O | Motility, adhesion, invasion, biofilm formation, expression of membrane proteins and metabolism genes | [50] | |
| Iron acquisition systems | iutA, iucC, iucD, aerJ, iucA, iucB, iroBCDEN, fyuA, sitABCD, mntH, feoB, irp2, ireA, eitABCD, fepC, chuA, bfr | Iron and manganese uptake from the host, adhesion, invasion, colonization, persistence, expression of virulence genes, resistance to environmental stresses | [36,37,51,52,53,54,55,56,57] |
| entE, entS, tolC | Invasion, colonization, persistence | [60] | |
| Protectins | iss, traT, ompT, kpsMT(K1), kpsMT(II), kpsMT(III), neuC, neuS, neuD, kfiC-K5, betA | Protect from serum bactericidal activity and phagocytosis, adhesion, invasion, intracellular survival, colonization, proliferation | [36,41,51,53,61] |
| YbjX, PagP | Resistance to serum and environmental stresses, invasion, intracellular survival | [62,63] | |
| OmpA | Intracellular survival | [64] | |
| wzy | Adhesion, invasion, intracellular survival, colonization | [65] | |
| waa L | Motility, resistance to phagocytosis and environmental stresses, adhesion, invasion, biofilm formation | [65] | |
| sod A | Protect against ROS-mediated host defenses, biofilm formation | [68] | |
| lpx M | Invasion, intracellular survival, colonization, regulation of expression of cytokine genes and nitric oxide production | [67] | |
| Toxins | hlyF, hlyA, hlyE, cdtB, cdtS, vat, sat, stx2f, astA, pic, EAST-1, espC, ace4/35 | Cell lysis and damage, induce host cell vacuolization, colonization, motility, biofilm formation, agglutination, formation of outer membrane vesicles | [35,38,39,41,51,53,69,70,71,72,73] |
| Other virulence and pathogenesis factors | |||
| Quorum-sensing system (AI-2) | LuxS, LsrABCDFGK, ptsI, Pfs | Motility, biofilm formation, adherence, invasion, colonization, intracellular survival, persistence, expression of virulence genes, cell damage | [10,74,75,76,77] |
| Secretion systems | DotU, CpxRA, IcmF, Hcp, ClpV, VrgG (Type VI) | Interbacterial competition, adhesion, invasion, intracellular survival, colonization, motility, biofilm formation, production of type 1 fimbriae, resistance to serum bactericidal activity, modulation of intracellular host responses (IL-18, IL-1β) | [11,80,81,82,83,84,85] |
| EtrA, YqeI, EivC (Type III) | Motility, intracellular survival, resistance to phagocytosis and serum bactericidal activity, proliferation, expression of fimbriae genes, downregulation of pro-inflammatory cytokines | [12,78,79] | |
| Two-component systems | PhoPQ, tolC | Biofilm formation, motility, adhesion, invasion, intracellular survival, systemic infection, expression of virulence genes and genes associated with flagellar assembly, ABC transporters, quorum sensing, and bacterial chemotaxis | [13,86,90] |
| BasSR | Biofilm formation, APEC virulence and colonization in vivo | [91] | |
| KdpDE | Expression of flagella-related genes, flagellum formation, motility and resistance to serum bactericidal activity | [87] | |
| RstAB, hdeD | Iron acquisition, acid resistance, intracellular survival, colonization | [88,92] | |
| BarA-UvrY | Adhesion, invasion, persistence, intracellular survival, resistance to serum bactericidal activity and oxidative stress, regulation of exopolysaccharide production and type 1 and P fimbriae | [89] | |
| Transcriptional regulators | AutA/AutR | Expression of K1 capsule and acid resistance systems, adaptive lifestyle change | [14] |
| FNR | Adhesion, invasion, expression of type 1 fimbriae and type VII secretion system, resistance to oxidative stress | [15] | |
| YjjQ | Flagellar motility | [93] | |
| McbR | Biofilm formation, response to H2O2 | [94] | |
| tyrR | Invasion, motility, intracellular survival | [37] | |
| RfaH | Invasion, intracellular survival, resistance to serum bactericidal activity | [95] | |
| Metabolism-associated genes | acs -yjcH-actP | Intracellular survival, proliferation, colonization, production of pro-inflammatory cytokines and nitric oxide | [96] |
| PotE, PotF | Colonization, adhesion | [16] | |
| NirC | Adhesion, colonization | [17] | |
| ArcA | Chemotaxis, motility | [18] | |
| Miscellaneous | OmpF, OmpC | Adhesion, invasion, colonization, proliferation | [100] |
| Prophage phiv142-3 (orf20) and phiv205-1 | Resistance to serum and environmental stresses, adhesion, invasion, intracellular survival, colonization, biofilm formation, formation of flagella and I fimbriae | [97,98,99] | |
| YicS | Motility, biofilm formation, invasion | [101] | |
| cpd B | Colonization | [102] | |
| pst B | Resistance to serum bactericidal activity and oxidative stress, colonization | [103] | |
| tmRNA-SmpB | Colonization, persistence, replication, intracellular survival | [104] | |
| mli C | Resistance to serum bactericidal activity | [105] | |
| malX, frz, cvaABC, cvi, cba, cib/cibI, cbi, cma, eaeA, sopB, yfcV, gad, mchBCF, mcmA, bor, air, eilA, celB, pabB, capU, cif, tir, tccp, nleB, iaL, cjrC, mig-14p | Unknown/not clearly known functions | [34,39,40,41,53,54,57,106,107,108] | |
| Genes essential for systemic infections and adaptation | metH, lysA, pntA, purL, serS, ybjE, ycdK (rutC), wcaJ, gspL, sdsR, irp2, eitD, ylbE, yjiY, tkt1, pilN, pilQ, tsh, hpb, TcfD, Z5222, waaO, waaY, iutA, iucA, iucD, iroC, ColE2, traK, traG, traT, SopA, psiA, hkaG, hkbV, hkbQ, Z3370, Int, CC0532, TM0427, YPO3000, rhsH, RSp0733, bioABFCD, rnfA, rfnE, gene encoding endonuclease III, creABCD, yehD, potF, flgE, tyrR, bfr | Systemic APEC infections and adaptation | [37,109,110,111] |
2.1. Adhesins
2.2. Invasins
2.3. Iron Acquisition Systems
2.4. Protectins
2.5. Toxins
2.6. Other Virulence and Pathogenesis Factors
Other virulence and pathogenesis factors of APEC include the QS system, transcriptional regulators, two-component systems, secretion systems, and genes associated with bacterial metabolism [10,11,12,13,14,15,16,17,18]. These factors assist in different processes of APEC pathogenesis/infection, including adhesion, invasion, colonization, persistence, interbacterial competitions, resistance to host defenses, and modulation of host immune responses [10,11,12,13,14,15,16,17,18], thereby facilitating the APEC proliferation and establishment of disease in the host.
2.7. Genes Essential for Systemic Infections and Adaptation in Chickens
Identifying the genes essential for systemic infections and adaptation is crucial to develop rational treatments against the infections. Nelwike et al. (2012) [109] investigated the APEC genes induced during systemic infections in chickens using RIVET (recombination-based in vivo expression technology). Genes involved in metabolism, cell envelope and integrity, transport systems, and virulence (metH, lysA, pntA, purL, serS, ybjE, ycdK (rutC), wcaJ, gspL, sdsR, irp2, eitD, ylbE, yjiY, tkt1, and phage-related genes) were upregulated in APEC isolated from infected chickens. Similarly, Dozois et al. (2003) [110] studied the APEC genes expressed in infected tissues in chickens using SCOTS (selective capture of transcribed sequences) technology. Genes involved in adherence (pilN, pilQ, tsh, hpb, TcfD, Z5222), LPS synthesis (waaO, waaY), iron acquisition (iutA, iucA, iucD, iroC), plasmid function (ColE2, traK, traG, traT, sopA, psiA), phage-related (hkaG, hkbV, hkbQ, Z3370, Int), and of unknown functions (CC0532, TM0427, YPO3000, rhsH, RSp0733) were highly expressed in APEC infected tissues. On the other hand, Zhang et al. (2019) [111] identified essential genes for APEC adaptation in chickens using the TraDIS (transposon-directed insertion site sequencing) strategy. Genes involved in metabolism, transport, regulation and stress response, RNA processing and translation, cell division and DNA replication, cell envelope biogenesis, and unknown functions were essential to cause disease in chickens. Particularly, genes involved in biotin synthesis (bioABFCD), Rnf electron transport complex (rnfA, rfnE, and gene nth encoding endonuclease III), and cre two-component system (creABCD) were important for the adaptation of APEC in chickens. In another study, genes identified as upregulated through microarray analysis (yehD, potF, flgE, tyrR, and bfr) in APEC isolated from chicken showing swollen head syndrome were essential for adhesion, invasion, survival inside macrophages, and motility [37]. These studies provide insights on APEC pathophysiological processes during systemic infections in chickens.
Overall, multiple virulence and pathogenesis factors of APEC are involved in causing colibacillosis in poultry. As a result of the involvement of multiple virulence and pathogenesis factors, there is a hindrance in developing therapeutics broadly effective against APEC infections. In-depth understanding of these factors as well as unraveling the new factors will help to develop the effective therapeutics against colibacillosis in poultry. Furthermore, several of these factors have coordinated and overlapping functions, which necessitates a holistic strategy to formulate an ideal anti-APEC therapeutics. For instance, developing therapeutics targeting iron acquisition systems [112], QS system [113], bacterial metabolism [114], and secretion systems [115] can provide solutions to mitigate APEC infections in poultry in the future.
Keywords: No change
This entry is adapted from the peer-reviewed paper 10.3390/pathogens10040467
