Endocrine-disrupting chemicals (EDCs) are hormonally active compounds in the environment that interfere with the body’s endocrine system and consequently produce adverse health effects. Despite persistent public health concerns, EDCs remain important components of common consumer products, thus representing ubiquitous contaminants to humans. While scientific evidence confirmed their contribution to the severity of Influenza A virus (H1N1) in the animal model, their roles in susceptibility and clinical outcome of the coronavirus disease (COVID-19) cannot be underestimated.
- endocrine disrupting chemicals
- COVID-19
- comorbid diseases
- immune dysfunction
1. Introduction
Endocrine-disrupting chemicals (EDCs) are ubiquitous in common consumer products, processed food, drinking water, food packaging, and plastic materials, and humans are regularly exposed to EDCs via oral, inhalation, transdermal, and parenteral routes [1][2]. The most common EDCs include bisphenols, phthalates, arsenic, pesticides, dioxins, and perfluorinated compounds. Due to their ability to interfere with hormone-driven processes and their toxicity, EDCs have become a major research focus in the last few decades. Research findings so far have confirmed EDCs as cogent contributors to the risk of the underlying comorbidities of chronic and infectious diseases [1][2][3][4][5][6][7]. EDCs such as arsenic and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) were reported to impair the host immune response to H1N1, leading to enhanced pulmonary inflammation and increased mortality [3][4]. Meanwhile, both COVID-19 and influenza have some similarities being contagious respiratory diseases [8].
Since its outbreak in late December 2019 in Wuhan city, China, the COVID-19 pandemic has spread to all continents [9]. As of 23 February 2021, there were 112,258,917 confirmed cases, 2,485,295 deaths, and 221 affected countries [10]. Early symptoms displayed by patients with COVID-19 include fever, cough, fatigue, and headache [11]. Older people and those with underlying comorbidities are prone to critical illness or death [12]. Among the first 41 patients with laboratory-confirmed COVID-19 as of 2 January 2020 at Jinyintan hospital, Wuhan, 32% had underlying comorbidities, i.e., diabetes mellitus, hypertension, and cardiovascular diseases [11]. According to another report, 269 (49.1%) of 548 patients with COVID-19 admitted to Tongji Hospital, China, from 26 January to 5 February 2020 were severely ill. Among these patients, 166 (30.3% of the total) and 83 (15.1% of total) had hypertension and diabetes as underlying comorbidities, respectively [5]. Similarly, 48% of 191 COVID-19 patients from Jinyintan and Wuhan Pulmonary Hospitals, China, were reported to have underlying comorbidities, with 30% having hypertension and 19% having diabetes [13]. Among 663 patients at Wuhan University Hospital from 11 January to 6 February 2020, a higher percentage (67.4%) of underlying comorbidities was the explanation for lack of improvement, severity, and mortality in COVID-19 disease [14]. Diabetes, high blood pressure, obesity, hypertension, low immunity, cardiovascular disease, and kidney and liver diseases as comorbidities result in extremely severe COVID-19 [12][13][15][16]. Meanwhile, long-term exposure to EDCs might be responsible for the development and progression of these diseases. Although there are variations in the percentage of patients with underlying medical conditions in different regions worldwide, metabolic, circulatory, and endocrine diseases are the most common comorbidities.
2. Sources of EDCs Exposure
EDCs are natural or synthetic compounds that can act as hormones by manipulating and compromising several mechanisms of the endocrine system to produce serious effect on the overall health of both humans and animals [17][18][19]. EDCs such as bisphenols (e.g., bisphenol A, BPA) and phthalates are essential chemicals used as raw materials in plastic industries [20]. Other groups of EDCs include dioxins, perchlorate, perfluoroalkyl, and polyfluoroalkyl substances, phytoestrogens, polybrominated diphenyl ethers (PBDE), polychlorinated biphenyls (PCB), and triclosan, which constitute components of many consumer and household products, including foods, resulting in widespread human exposure [21][22]. A search of the literature demonstrated that the vast majority of EDCs are widely and increasingly being used worldwide. Derivatives of BPA (bisphenol S, bisphenol F, and bisphenol E) are used in the production of polycarbonate plastics, epoxy resins, food packaging, dental sealants, and thermal receipts [23][24][25]. High-molecular-weight phthalates such as di (2-ethylhexyl) phthalate (DEHP), di-isononyl phthalate di-isodecyl phthalate, and benzylbutyl phthalate are used in making polyvinyl chloride (PVC) plastics, medical devices, pharmaceutical coatings, food packaging, car interiors, drinking straws, and adhesives [26]. Low-molecular-weight phthalates such as diethyl phthalate, di-n-butyl phthalate, and di-iso-butyl phthalate are used in making perfumes, deodorants, nail polish, and insecticides [26]. PBDE, perchlorate, triclosan, and polychlorinated biphenyls are used in making furniture foam, fireworks, liquid body wash, and hydraulic fluids, respectively [27]. As the most common EDCs, BPA and phthalates can be ingested along with food and beverages packaged in containers containing them. The ubiquitous distribution of EDCs makes it possible for humans to be exposed to EDCs via the transdermal, oral, inhalation, and parenteral routes [28][29]. A summary of commonly used EDCs and their exposure sources is compiled in Figure 1.
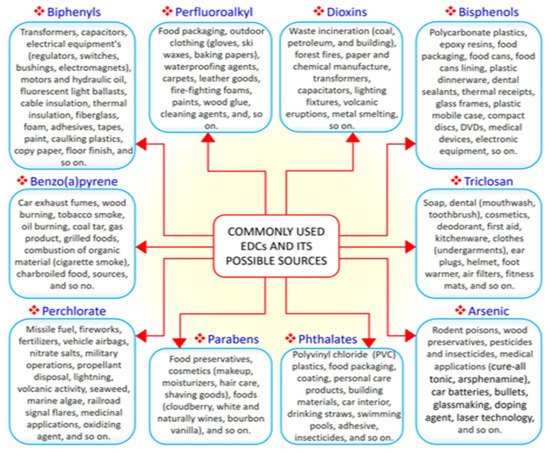
Figure 1. The 10 commonly used endocrine-disrupting chemicals (EDCs) and their common sources.
3. EDC-Related Diseases
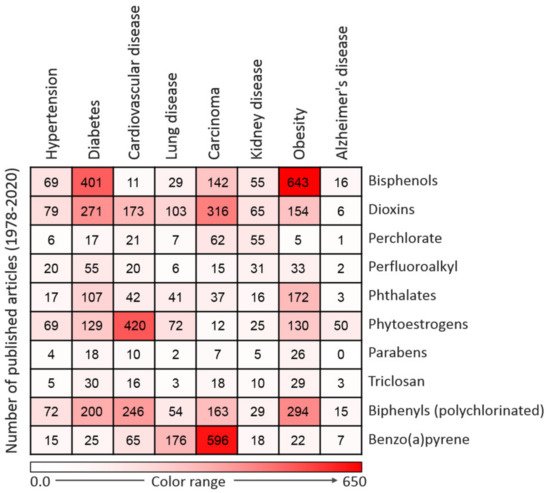
EDCs have notable effects on biological systems. Exposure to EDCs alters the endocrine system of the body and causes serious metabolic, neurological, cardiovascular, and immunological effects in humans and animals, including wildlife [30]. Generally, EDCs via binding with estrogen receptors can activate transcription factors such as protein-1 (AP-1), nuclear factor-kappa B (NF-κB), and specificity factor-1 (Sp1), which are involved in the pathogenesis of inflammation which accounts for the onset of many chronic and comorbid diseases [30][31][32]. The synthesis of natural hormones may be obstructed and inhibited by EDCs [30]. The effect of exposure to EDCs during the critical stage of development could be latent until adulthood [31]. It has been proven that EDCs not only affect the exposed person but also their offspring and successive generations [32]. Research has established that exposure to EDCs is positively correlated with the escalated emergence of cancer, obesity, Parkinson’s disease, and other diseases [31][33][34]. These effects compelled the Endocrine Society to release a publication in 2009 showing EDCs as important public health concerns [35]. A summary of major EDC-related diseases is presented in Figure 2.
Figure 2. Heatmap representing the number of articles published on the 10 commonly used endocrine-disrupting chemicals (EDCs) in association with particular diseases. Data were collected by a thorough search of the Web of Science database. Only peer-reviewed papers published in English from 1978 to 2020 on specific EDCs and diseases were included.
3.1. Metabolic Disorders and Obesity
Recently, a number of studies reported an association between EDC exposure and the stimulation of adipogenesis and weight gain [36]. Obesogens EDCs can promote weight gain through the proliferation and subsequent storage of fat cells [37].Obesogens such as BPA, PCBs, dioxins, and phthalates can also promote weight gain by compromising energy homeostasis and the basal metabolic rate [36][38]. At a molecular level, obesogens EDCs act by interfering with nuclear transcriptional regulators that control lipid flux and/or adipocyte proliferation/differentiation, especially the peroxisome proliferator-activated receptors (PPARα, PPAR-δ, and PPAR-γ) and steroid hormone receptors. PPARs act by heterodimerization with retinoid X receptors (RXRs), and the activation of RXR-PPARγ potentiates the differentiation of adipocyte progenitors and preadipocytes in adipose tissue. Consequently, fat/lipid biosynthesis and storage are promoted and finally result in obesity [36][38]. Obesity-related health conditions are prevalent in many industrialized countries where EDCs are produced and used in large quantities [39]. In 2013, 90 million (28.6%) cases of obesity were found in the US population of 315 million, and 78 million of these cases were found in adults [39]. It has been extrapolated that approximately 40% of the world’s population will have obesity by 2030 [40]. Obesity is known as a predisposing factor for several health conditions, including diabetes and hypertension [40]. An important cause of insulin resistance (IR) is oxidative stress, and elevated oxidative stress is induced by obesity via the excessive generation of mitochondrial energy [41]. Further, IR results in elevated circulating glucose levels, which in turn worsens the generation of oxidative stress [42], and type 2 diabetes and vascular disease may result from this vicious cycle [40]. A recent study also found a positive correlation between Alzheimer’s disease and IR [43].
3.2. Diabetes
To address the public concern that exposure to EDCs could contribute to widespread diabetes, researchers in the field of EDC research have conducted studies to investigate the association between EDC exposures and diabetes. Currently, there is confirmed evidence that diabetes is related to exposure to EDCs such as bisphenols, pesticides, and dioxins [44][45][46]. The same EDCs have been indicted in the development and progression of diabetes and obesity [36][38][47]. In a study conducted by Lee et al. [48] to investigate the association between persistent organic pollutants (a group of EDCs) and the prevalence of diabetes among 2016 adult participants, the serum concentration of persistent organic pollutants was found to be positively correlated with diabetes prevalence. In addition, exposure to many other groups of EDCs, such as polychlorinated biphenyls, bisphenols, dioxins, phthalates, and organochlorinated pesticides, has been reported to be associated with the risk of diabetes [49]. Persistent organic pollutants, BPA, dichlorodiphenyltrichloroethane (DDT), and phthalates have all been documented to play a diabetogenic role [50][51][52]. Association between EDC exposures and diabetes has also been demonstrated in experimental studies. Reduced insulin levels were observed in adult mice exposed to DDT during the perinatal window of development [53]. A similar study in which mice were exposed to BPA in utero resulted in the impairment of insulin secretion and glucose tolerance [54]. The molecular mechanism of EDCs action involves binding to the estrogen receptors α and β and thereby acting like estrogen. Long-term exposure to xenoestrogen EDCs hyperactivates the β-cells, leading to hyperinsulinemia. Consequently, there is a development of a condition known as insulin resistance/glucose intolerance, which is a significant cause of diabetes [44].
3.3. Hypertension and Cardiovascular Diseases
Hypertension (high blood pressure) is prevalent worldwide, mostly in older adults but also in individuals of different ages, and is considered to be among the leading causes of death in developed countries [55]. Hypertension related to hormone imbalance is common among humans. Vasodilation can be induced by estrogen via both genomic and non-genomic pathways. Endocrine disruptors are estrogenic and have been identified as risk factors contributing to the onset of hypertension [55]. The urinary concentration of BPA was shown to be positively correlated with hypertension in a survey conducted on 2588 individuals by the Thai National Health Examination Survey 2009 [56]. Another study conducted from 2008 to 2010 in Seoul, South Korea, reported a positive correlation between urinary BPA concentrations and blood pressure in 521 elderly citizens [57]. Urinary concentrations of BPA were reported to be correlated with increased diastolic blood pressure in 39 boys recruited from the Children Medical Center of Dayton, Ohio [58]. The evaluation of urinary BPA concentrations in 1380 participants of the 2003–2004 National Health and Nutritional Examination Survey (NHANES) also showed a positive correlation between elevated levels of urinary BPA and hypertension [59]. Consistently, cross-sectional studies have confirmed the effect of BPA on cardiovascular diseases. The analysis of NHANES data from 2003 to 2004 revealed a high correlation between the urinary concentration of BPA and cardiovascular diseases including myocardial infarction and coronary heart disease [60][61]. A urinary BPA concentration of 4.56 ng/mL significantly increased the risk of coronary artery disease [62]. Apart from the direct effect, hypertension is a prominent risk factor for cardiovascular diseases [63]. Since BPA affects blood pressure, it could cause cardiovascular disease via increased blood pressure. BPA can further affect the cardiovascular system because it is estrogenic; estrogen receptors are found in cells of the cardiovascular system, and estrogen is involved in vasodilation [61]. Estrogen is active both on vascular smooth muscle and endothelial cells where functional estrogen receptors have been found. By acting on vascular smooth muscle cells, EDCs activate K+ channels, leading to cell hyperpolarization, increase aortic stiffness, potentiate endothelial vasodilator function, and block the activation of Ca2+ channels, resulting in decreased intracellular Ca2+ concentration. Xenoestrogen EDCs promote vasodilation in humans by stimulating prostacyclin and nitric oxide synthesis and decreasing the production of vasoconstrictor agents [61]. Similarly, the onset of coronary heart disease, high blood pressure, and atherosclerosis in humans has been attributed to exposure to other EDCs [64].
3.4. Kidney Diseases
The nephrotoxic effect of most endocrine disruptors is a public health concern. Epidemiological studies have established a positive association between renal diseases and urinary BPA concentration in humans [65][66]. A cross-sectional study involving 3455 Chinese participants indicated that an average urinary BPA concentration of 0.81 ng/mL was correlated with an elevated risk of albuminuria [65]. Similarly, another cross-sectional study involving 710 children in the USA indicated that an average urinary BPA concentration of 0.91 mg/g is associated with albuminuria [66]. A renal function analysis of 184 children aged 10 years exposed to DEHP contaminated food revealed a significant association between exposure to DEHP and an elevated urine albumin/creatinine ratio [67]. The study further found that highly exposed children (with an average daily DEHP intake of 0.05 mg/kg/day) were 10.39% prone to the risk of microalbuminuria. Female mice exposed to 1500 and 6000 ppm of DEHP had a significantly higher proportion of chronic progressive nephropathy (CPN) cases than those in the control group [68]. In the same study, male and female mice exposed to the same concentration of DEHP experienced a reduction in kidney weight [68]. CPN in male rats was reported to be aggravated following exposure to 12,500 ppm of DEHP [68]. A separate report indicated that exposure to 3147 mg/kg/day of DEHP in mice resulted in the degeneration of the renal tubule and reduced kidney weight [69]. A rat model study, in which male Wistar rats were exposed to 50, 100, and 150 mg/kg of BPA for 5 weeks, found that BPA caused proteinuria, glomerular injuries, elevated serum, and urea creatinine in a dose-dependent manner [70]. EDCs, through steroidogenesis, accelerate kidney estrogen metabolism and stimulate the activity of cytochrome p-450 aromatase, resulting in oxidative stress. Some EDCs are competent to act directly on the kidney mitochondria, resulting in mitochondrial oxidative stress, dysfunction, and subsequently, whole organ damage [70]. Many EDCs such as BPA display nephrotoxicity and function as indicators for renal disease [71]. The current chronic kidney disease prevalence of 10–15% in the general population worldwide and the fact that EDCs contribute to this prevalence imply that EDCs constitute a threat to human wellbeing [72][73].
3.5. Cancer
Approximately 9.6 million deaths and 18.1 million new cases of various types of cancer were reported in 185 countries in 2018 [74]. The relationship between EDCs and cancer has been established for over a decade. Investigations have found several EDCs to be carcinogens that can promote the onset and progression of cancer through their hormone-like activities [75][76][77]. Recently, the development of cancer has been linked to microRNAs which are known to negatively regulate the expression of genes [78]. Estrogen-regulated onco-miR-21 has been shown to play a vital role in the development of breast cancer [79]. Dioxins, DEHP, and BPA can stimulate estrogen receptors, thus contributing to the development of estrogen-dependent cancers such as prostate and breast cancers [80]. EDCs can bind many nuclear receptors, such as estrogen receptors (ERα and β), GPR30, androgen receptor (AR), thyroid hormone receptors (TRα and β), estrogen-related receptor gamma (ERRγ) and glucocorticoid receptor (GR). The binding of EDCs to ER increases the proliferation and migration of several cancer cell types through a pathway involving Stat3 and ERK1/2 [75][76][77][78][79][80]. In a study conducted to evaluate the relationship between endocrine disruptors and the risk of thyroid cancer in 960 individuals (462 thyroid cancer patients and 498 control), increased risk of thyroid cancer was observed in persons who were exposed to EDCs compared to their control counterparts [81]. An investigation of the association between circulating serum EDC levels and mammographic breast density (an indicator of breast cancer risk) among 264 women from mammography clinics indicated that serum concentrations of BPA and mono-ethyl phthalate were positively correlated with mammographic breast density [82]. In another study, phytoestrogens, PCB, and dioxins were linked to the development of breast cancer, while arsenic and PCB were reported to significantly contribute to the incidence of prostate cancer [6][83].
3.6. Lung Diseases
The involvement of several EDCs in the development of human diseases via various routes and mechanisms has been established by several studies [19][84][85]. Exposure to EDCs can activate ERK1/2 via GPER/EGFR. Consequently, GPER/ERFR/ERK1/2 mediates the upregulation of matrix metalloproteinases (MMPs), collectively known as the gelatinases, which are generally crucial in inflammatory, infectious pathogenesis, neoplastic diseases, and the migration of lung cancer [31][84][85]. The insecticides used in many residential buildings, chemical components of the building, and furnishing materials are EDC sources of indoor exposure [86][87]. Low-level exposure to indoor EDCs is related to an increased risk of asthma. A cross-sectional study found that the risk of developing asthma was significantly increased in 815 pupils exposed to a mixture of EDCs (hexane, styrene, cyclohexanone, butylated hydroxytoluene, and 2-butoxyethanol) [88]. Cleaning agents and air fresheners containing EDCs have been identified as sources by which building occupants and cleaning personnel are exposed to a large number of airborne chemicals, consequently developing lung problems [89]. Exposure to approximately 3.4–17 mg/m3 of sodium tripolyphosphate and 14 mg/m3 of volatile organic compounds following carpet cleaning was reported to result in asthma and seizure in a 42-year-old woman [90]. In a separate occupational exposure case, all-female nurses of a hospital showed symptoms of asthma after handling a disinfectant solution containing EDCs [91]. A total of 2414 Finnish female cleaners developed asthma via exposure to cleaning agents, with a risk factor of 1.50 [92]. A similar finding was reported among Spanish cleaners; 28% of the cleaners developed asthma via exposure to EDC-containing kitchen cleaning agents and furniture polishing chemicals [93]. It has been demonstrated that a BPA concentration of 10−4 M promoted the proliferation and migration of A549 human lung cancer cells [94]. A risk of respiratory symptoms was linked to occupational exposure to PVC-containing fumes and residential exposure to PVC-contaminated dust [95]. Another human study indicated a positive correlation between asthma in children and the phthalate component of building dust [96]. In a study conducted on 56 children with asthma in Seoul, South Korea, urinary concentrations of phthalates (mono-[2-ethyl-5-hydroxyhexyl] phthalate and mono-[2-ethyl-5-oxohexyl] phthalate, metabolites of DEHP, and mono-n-butyl phthalate, a metabolite of di-n-butyl phthalate) were correlated with decreased pulmonary function, airway inflammation, and increased levels of fractional exhaled nitric oxide [97]. In addition, a clinical study aimed at investigating the association between the urinary concentration of EDCs and the function of the lung indicated that urinary concentrations of BPA and phthalates correlated with the impairment of lung function and oxidative stress in 411 persons aged > 58 years recruited for the study [98].
3.7. Neurodegenerative Diseases
Neurodegenerative diseases including Alzheimer’s disease affect people worldwide, especially older people. The number of Alzheimer’s disease cases is projected to increase to approximately 106 million in the next 30 years [99]. Although 70% of Alzheimer’s disease risk is attributed to genetics, environmental factors including EDC exposures account for the remaining 30% [100]. Insecticides, pesticides, dioxins, bisphenols, phthalates, and parabens are groups of endocrine disruptors that have been implicated in the development of Alzheimer’s disease. The nervous system of insect pests is the target of various pesticides; likewise, these chemicals are neurotoxic to humans [101]. Neurological diseases including Alzheimer’s disease have been attributed to exposures to pesticides [102]. A study that examined 7321 PCB-exposed workers found that serum PCB levels in exposed workers were approximately 10 times higher than those in community controls. Dementia, Parkinson’s disease, and neurological disease-related deaths were reported among highly exposed women [103]. An investigation into the link between pesticide exposure and Parkinson’s disease revealed a significant correlation: a significantly increased risk of Parkinson’s disease was observed in 13 of 23 cases, with a risk estimate of 2.4 [104]. An analysis of hospital records between 1998 and 2005 indicated that cases of Parkinson’s disease and Alzheimer’s disease were higher among people dwelling in an area of high pesticide use [105]. Following the investigation of a possible link between Alzheimer’s disease and dementia and exposure to pesticides among 5092 persons dwelling in Cache County, Utah, USA, a significantly high correlation was found between exposure to organophosphates and Alzheimer’s disease [106]. EDCs act on the pituitary gland or bind with estrogen/G-protein-coupled receptors involved in neurotransmission and, consequently, affect the central nervous system’s central dopamine neurons and monoaminergic neurons [75][76][77][78][79][80][84][85].
3.8. Immune Function
EDCs not only disrupt hormone activities but are also known to alter the function of the immune system. Human epidemiological studies have indicated a clear relationship between the development of allergic diseases and exposure to EDCs [107][108]. Evaluation of the effect of EDC exposure on infants was performed by comparing 73 bottle-fed and 98 breastfed children, correlating disease history within the early postnatal stage and the organochlorine concentration in milk. The result showed that prenatal exposure to dichlorodiphenyldichloroethylene and hexachlorobenzene was related to the risk of otitis media [109]. A study conducted to investigate the effect of gestational exposure to PCBs reported that the antibody response to diphtheria toxoid in 119 children exposed to PCBs during pregnancy decreased by 24.4% at 18 months of age. The same study confirmed that perinatal exposure to PCBs resulted in a 16.5% decrease in tetanus toxoid antibody response at 7 years of age in 129 children examined during the study [110]. A study conducted to assess the effect of BPA and triclosan on immune parameters in the US population indicated that the urinary concentration of BPA was correlated with elevated cytomegalovirus antibody titers, while the urinary concentration of triclosan showed a positive correlation with allergy and hay fever diagnoses. The author concluded that BPA and triclosan suppressed human immune function [111]. Lipophilic EDCs have also been reported to cause immunological disorders in infants [112]. An inverse association was reported between organochlorine pesticides and T helper cell type 1 in 31 randomly recruited women from Western Australia [113]. The suppression of T helper cells and skewed balance in Th1/Th2 are mechanisms by which EDCs compromise immune function [31]. Another study involving the analysis of whole blood samples from 349 children exposed to PCB indicated that postnatal exposure to PCB resulted in a fluctuation in lymphocyte subsets, suggesting the impairment of the postnatal immune system [114]. It is also worth noting that the complete lockdown implemented in most countries to contain the spread of the virus has forced many people to stock their houses with canned food, junk food, and food items preserved with potential EDCs. Consequently, many people may develop immunosuppression and be prone to severe SARS-CoV-2 infection owing to long-term exposure to EDCs.
4. Current Knowledge on EDCs and COVID-19 Risks
Since COVID-19’s emergence in late 2019, EDCs have been speculated to be contributors to its risks [115]. The role of long-term exposure to toxic chemicals in COVID-19 clinical outcomes was reported to be grossly neglected, leading to the one sided biological approach of containment while the toxicological approach is abandoned [116]. Additionally, the spread and mortality rate of COVID-19 were presented as an opportunity to reassess the correlation between exposure to anthropogenic pollutants and pandemics [117]. Recently, a computational systems biology approach was used to study the relationship between EDCs and COVID-19 severity and identified the T-helper cell 17 (Th17) and the advanced glycation end products/receptor for advanced glycation end products (AGE/RAGE) pathways as principal targets through which EDCs could contribute to COVID-19 severity [118]. A non-mechanistic study that analyzed the urine and serum concentrations of Per- and poly-fluoroalkyl substances (PFASs) found a positive association between urinary levels of perfluorooctanesulfonic acid (PFOS) (odds ratio: 2.29 (95% CI: 1.52–3.22)), perfluorooctanoic acid (PFOA) (2.91, (1.95–4.83)), and total PFASs (Σ (12) PFASs) (3.31, (2.05–4.65)) with the risk of COVID-19 infection [119]. These preliminary studies provide insight into how EDCs can influence the clinical outcome of COVID-19 disease. However, the relationship between EDCs and COVID-19 risks still requires a comprehensive investigation.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22083939
References
- Matuszczak, E.; Komarowska, M.D.; Debek, W.; Hermanowicz, A. The impact of bisphenol A on fertility, reproductive system, and development: A review of the literature. Int. J. Endocrinol. 2019, 2019, 4068717.
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA J. 2015, 13, 3978.
- Kozul, C.D.; Ely, K.H.; Enelow, R.I.; Hamilton, J.W. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environ. Health Perspect. 2009, 117, 144–1447.
- Warren, T.K.; Mitchell, K.A.; Lawrence, B.P. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses the humoral and cell-mediated immune responses to influenza A virus without affecting cytolytic activity in the lung. Toxicol. Sci. 2000, 56, 114–123.
- Wehbe, Z.; Nasser, S.A.; El-Yazbi, A.; Nasreddine, S.; Eid, A.H. Estrogen and Bisphenol A in Hypertension. Curr. Hypertens. Rep. 2020, 22, 23.
- De Coster, S.; van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public Health 2012, 2012, 713696.
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155.
- Centers for Disease Control and Prevention. Similarities and Differences between Flu and COVID-19. Available online: (accessed on 27 May 2020).
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 106–1069.
- WHO Coronavirus Disease (COVID-19) Dashboard. Available online: (accessed on 22 May 2020).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118.
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062.
- Zhang, J.; Wang, X.; Jia, X.; Li, J.; Hu, K.; Chen, G.; Wei, J.; Gong, Z.; Zhou, C.; Yu, H.; et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin. Microbiol. Infect. 2020, 26, 767–772.
- Li, G.; Hu, R.; Gu, X. A close-up on COVID-19 and cardiovascular diseases. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1057–1060.
- Bornstein, S.R.; Dalan, R.; Hopkins, D.; Mingrone, G.; Boehm, B.O. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020, 16, 297–298.
- Ben Maamar, M.; Lesné, L.; Desdoits-Lethimonier, C.; Coiffec, I.; Lassurguère, J.; Lavoué, V.; Deceuninck, Y.; Antignac, J.P.; Le Bizec, B.; Perdu, E.; et al. An investigation of the endocrine-disruptive effects of bisphenol a in human and rat fetal testes. PLoS ONE 2015, 10, e0117226.
- Desdoits-Lethimonier, C.; Lesné, L.; Gaudriault, P.; Zalko, D.; Antignac, J.P.; Deceuninck, Y.; Platel, C.; Dejucq-Rainsford, N.; Mazaud-Guittot, S.; Jégou, B. Parallel assessment of the effects of bisphenol A and several of its analogs on the adult human testis. Hum. Reprod. 2017, 32, 1465–1473.
- Henley, D.V.; Korach, K.S. Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology 2006, 147, S25–S32.
- Genoa, R.W.; Jodi, A.F. Bisphenol A and Phthalates: How environmental chemicals are reshaping toxicology. Toxicol. Sci. 2018, 166, 246–249.
- Cao, X.L.; Zhao, W.; Churchill, R.; Hilts, C. Occurrence of di-(2-ethylhexyl) adipate and phthalate plasticizers in samples of meat, fish, and cheese and their packaging films. J. Food Prot. 2014, 77, 610–620.
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, January 2019, Volume One. Available online: (accessed on 22 May 2020).
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005, 84, 249–259.
- Clark, E. Sulfolane and sulfones. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: New York, NY, USA, 2006.
- Office of Environmental Health Hazard Assessment. Potential Designated Chemicals: P, p’-Bisphenols and Diglycidyl Ethers of p, p-Bisphenols. 2012. Available online: (accessed on 25 July 2020).
- Pacyga, D.C.; Sathyanarayana, S.; Strakovsky, R. Dietary Predictors of Phthalate and Bisphenol Exposures in Pregnant Women. Adv. Nutr. 2019, 10, 803–815.
- National Institute of Environmental Health Science. Endocrine Disruptors. Available online: (accessed on 19 May 2020).
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A new chapter in the bisphenol a story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015, 103, 11–21.
- Zalko, D.; Jacques, C.; Duplan, H.; Bruel, S.; Perdu, E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere 2011, 82, 424–430.
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215.
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ. Int. 2019, 125, 350–364.
- Rahman, M.S.; Kwon, W.S.; Karmakar, P.C.; Yoon, S.J.; Ryu, B.Y.; Pang, M.G. Gestational Exposure to Bisphenol-A Affects the Function and Proteome Profile of F1 Spermatozoa in Adult Mice. Environ. Health Perspect. 2017, 125, 238–245.
- Cohn, B.A.; Wolff, M.S.; Cirillo, P.M. DDT and breast cancer in young women: New data on the significance of age at exposure. Environ. Health Perspect. 2007, 115, 1406–1414.
- Vandenberg, L.N.; Maffini, M.V.; Schaeberle, C.M.; Ucci, A.A.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod. Toxicol. 2008, 26, 210–219.
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev. 2009, 30, 293–342.
- Janesick, A.S.; Blumberg, B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Res. C Embryo Today 2011, 93, 34–50.
- Darbre, P.D. Endocrine disruptors and obesity. Curr. Obes. Rep. 2017, 6, 18–27.
- Grun, F.; Blumberg, B. Endocrine disrupters as obesogens. Mol. Cell. Endocrinol. 2009, 304, 19–29.
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil. Steril. 2017, 107, 833–839.
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437.
- Salmon, A.B. Beyond diabetes: Does obesity-induced oxidative stress drive the aging process? Antioxidants 2006, 5, 24.
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072.
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1078–1089.
- Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 7, 346–353.
- Alonso-Magdalena, P.; Ropero, A.B.; Soriano, S.; Quesada, I.; Nadal, A. Bisphenol-A: A new diabetogenic factor? Hormones 2010, 9, 118–126.
- Hectors, T.L.; Vanparys, C.; van der Ven, K.; Martens, G.A.; Jorens, P.G.; Van Gaal, L.F.; Covaci, A.; De Coen, W.; Blust, R. Environmental pollutants and type 2 diabetes: A review of mechanisms that can disrupt beta cell function. Diabetologia 2011, 54, 1273–1290.
- Grun, F.; Blumberg, B. Minireview the case for obesogens. Mol. Endocrinol. 2009, 23, 1127–1134.
- Lee, D.H.; Lee, I.K.; Song, K.; Steffes, M.; Toscano, W.; Baker, B.A.; Jacobs, D.R. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: Results from the National Health and Examination Survey 1999–2002. Diabetes Care 2006, 29, 1638–1644.
- Ruiz, D.; Becerra, M.; Jagai, J.S.; Ard, K.; Sargis, R.M. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care 2018, 41, 193–205.
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602.
- Marroqui, L.; Tuduri, E.; Alonso-Magdalena, P.; Quesada, I.; Nadal, A.; Dos Santos, R.S. Mitochondria as target of endocrine-disrupting chemicals: Implications for type 2 diabetes. J. Endocrinol. 2018, 239, R27–R45.
- Cardenas, A.; Gold, D.R.; Hauser, R.; Kleinman, K.P.; Hivert, M.F.; Calafat, A.M.; Ye, X.; Webster, T.F.; Horton, E.S.; Oken, E. Plasma Concentrations of Per- and Polyfluoroalkyl Substances at Baseline and Associations with Glycemic Indicators and Diabetes Incidence among High-Risk Adults in the Diabetes Prevention Program Trial. Environ. Health Perspect. 2017, 125, 107001.
- Uslu, U.; Sandal, S.; Cumbul, A.; Yildiz, S.; Aydin, M.; Yilmaz, B. Evaluation of estrogenic effects of polychlorinated biphenyls and organochlorinated pesticides using immature rat utero trophic assay. Hum. Exp. Toxicol. 2013, 32, 476–482.
- Garcia-Arevalo, M.; Alonso-Magdalena, P.; Rebelo Dos Santos, J.; Quesada, I.; Carneiro, E.M. Exposure to bisphenol-a during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS ONE 2014, 9, e100214.
- Yan, S.; Chen, Y.; Dong, M.; Song, W.; Belcher, S.M.; Wang, H.S. Bisphenol A and 17beta-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS ONE 2011, 6, e25455.
- Aekplakorn, W.; Chailurkit, L.O.; Ongphiphadhanakul, B. Association of serum bisphenol a with hypertension in thai population. Int. J. Hypertens. 2015, 2015, 594189.
- Bae, S.; Kim, J.H.; Lim, Y.H.; Park, H.Y.; Hong, Y.C. Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension 2012, 60, 786.
- Khalil, N.; Ebert, J.R.; Wang, L.; Belcher, S.; Lee, M.; Czerwinski, S.A.; Kannan, K. Bisphenol A and cardiometabolic risk factors in obese children. Sci. Total Environ. 2014, 470–471, 726–732.
- Shankar, A.; Teppala, S. Urinary bisphenol A and hypertension in a multiethnic sample of US adults. J. Environ. Public Health 2012, 2012, 481641.
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310.
- Han, C.; Hong, Y. Bisphenol A, Hypertension, and Cardiovascular Diseases: Epidemiological, Laboratory, and Clinical Trial Evidence. Curr. Hypertens. Rep. 2016, 18, 11.
- Melzer, D.; Osborne, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.; Wareham, N.J.; et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 2012, 125, 1482–1490.
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99.
- Mariana, M.; Feiteiro, J.; Verde, I.; Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 2016, 94, 758–776.
- Li, M.; Bi, Y.; Qi, L.; Wang, T.; Xu, M.; Huang, Y.; Xu, Y.; Chen, Y.; Lu, J.; Wang, W.; et al. Exposure to bisphenol A is associated with low-grade albuminuria in Chinese adults. Kidney Int. 2012, 81, 1131–1139.
- Trasande, L.; Attina, T.M.; Trachtman, H. Bisphenol A exposure is associated with low-grade urinary albumin excretion in children of the United States. Kidney Int. 2013, 83, 741–748.
- Tsai, H.J.; Chen, B.; Wu, C.F.; Wang, S.; Huang, P.; Tsai, Y.; Chen, M.; Ho, C.; Chao, A.; Hsiung, C.A.; et al. Intake of phthalate-tainted foods and microalbuminuria in children: The 2011 Taiwan food scandal. Environ. Int. 2016, 89–90, 129–137.
- David, R.M.; Moore, M.R.; Finney, D.C.; Guest, D. Chronic toxicity of di(2-ethylhexyl) phthalate in mice. Toxicol. Sci. 2000, 58, 377–385.
- Wood, C.E.; Jokinen, M.P.; Johnson, C.L.; Olson, G.R.; Hester, S.; George, M.; Chorley, B.N.; Carswell, G.; Carter, J.H.; Wood, C.R.; et al. Comparative time course profiles of phthalate stereoisomers in mice. Toxicol. Sci. 2014, 139, 21–34.
- Kobroob, A.; Peerapanyasut, W.; Chattipakorn, N.; Wongmekiat, O. Damaging Effects of Bisphenol A on the Kidney and the Protection by Melatonin: Emerging Evidences from In Vivo and In Vitro Studies. Oxid. Med. Cell. Longev. 2018, 2018, 3082438.
- Gowder, S.J.T. Nephrotoxicity of Bisphenol A (BPA)—An Updated Review. Curr. Mol. Pharmacol. 2013, 6, 163–172.
- Koch, C.A.; Diamanti-Kandarakis, E. Introduction to endocrine disrupting chemicals—Is it time to act? Rev. Endocr. Metab. Disord. 2015, 6, 269–270.
- Lerma, E.V.; Koch, C.A. Nephroendocrinology: When endocrinology meets nephrology. Rev. Endocr. Metab. Disord. 2017, 18, 1–3.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Del Pup, L.; Mantovani, A.; Cavaliere, C.; Facchini, G.; Luce, A.; Sperlongano, P.; Caraglia, M.; Berretta, M. Carcinogenetic mechanisms of endocrine disruptors in female cancers (Review). Oncol. Rep. 2016, 36, 603–612.
- Soto, A.M.; Sonnenschein, C. Environmental causes of cancer: Endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 2010, 6, 363–370.
- Wogan, G.N.; Hecht, S.S.; Felton, J.S.; Conney, A.H.; Loeb, L.A. Environmental and chemical carcinogenesis. Semin. Cancer Biol. 2004, 14, 473–486.
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261.
- Tilghman, S.L.; Bratton, M.R.; Segar, H.C.; Martin, E.C.; Rhodes, L.V.; Li, M.; McLachlan, J.A.; Wiese, T.E.; Nephew, K.P.; Burow, M.E. Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PLoS ONE 2012, 7, e32754.
- Park, M.A.; Hwang, K.A.; Choi, K.C. Diverse animal models to examine potential role(s) and mechanism of endocrine disrupting chemicals on the tumor progression and prevention: Do they have tumorigenic or anti-tumorigenic property. Lab. Anim. Res. 2011, 27, 265–273.
- Zeng, F.; Lerro, C.; Lavoué, J.; Huang, H.; Siemiatycki, J.; Zhao, N.; Ma, S.; Deziel, N.C.; Friesen, M.C.; Udelsman, R.; et al. Occupational exposure to pesticides and other biocides and risk of thyroid cancer. Occup. Environ. Med. 2017, 74, 502–510.
- Sprague, B.L.; Trentham-Dietz, A.; Hedman, C.J.; Wang, J.; Hemming, J.D.; Hampton, J.M.; Buist, D.S.; Aiello Bowles, E.J.; Sisney, G.S.; Burnside, E.S. Circulating serum xenoestrogens and mammographic breast density. Breast Cancer Res. 2013, 15, R45.
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258.
- Polyzos, S.A.; Kountouras, J.; Deretzi, G.; Zavos, C.; Mantzoros, C.S. The emerging role of endocrine disruptors in pathogenesis of insulin resistance: A concept implicating nonalcoholic fatty liver disease. Curr. Mol. Med. 2012, 12, 68–82.
- Darbre, P.D. Endocrine Disruption and Human Health; Academic Press: Cambridge, MA, USA, 2015; pp. 27–45.
- Rudel, R.A.; Perovich, L.J. Endocrine disrupting chemicals in indoor and outdoor air. Atmos. Environ. 2009, 43, 170–181.
- Butte, W.; Heinzow, B. Pollutants in house dust as indicators of indoor contamination. Rev. Environ. Contam. Toxicol. 2002, 175, 1–46.
- Paciência, I.; Cavaleiro, R.J.; Silva, D.; Carla Martins, C.; Francisca Mendes, F.; Farraia, M.; Delgado, L.; Fernandes, E.O.; Padrão, P.; Moreira, P.; et al. Exposure to indoor endocrine-disrupting chemicals and childhood asthma and obesity. Allergy 2019, 74, 1277–1291.
- Nazaroff, W.W.; Weschler, C.J. Cleaning products and air fresheners: Exposure to primary and secondary air pollutants. Atmos. Environ. 2004, 38, 2841–2865.
- Lynch, R.M. Modeling of exposure to carpet-cleaning chemicals preceding irritant-induced asthma in one patient. Environ. Health Perspect. 2000, 108, 911–913.
- Purohit, A.; Kopferschmitt-Kubler, M.C.; Moreau, C.; Popin, E.; Blaumeiser, M.; Pauli, G. Quaternary ammonium compounds and occupational asthma. Int. Arch. Occup. Environ. Health 2000, 73, 423–427.
- Karjalainen, A.; Martikainen, R.; Karjalainen, J.; Klaukka, T.; Kurppa, K. Excess incidence of asthma among Finnish cleaners employed in different industries. Eur. Respir. J. 2002, 19, 90–95.
- Zock, J.P.; Kogevinas, M.; Sunyer, J.; Almar, E.; Muniozguren, N.; Payo, F.; Sánchez, J.L.; Antó, J.M. Asthma risk, cleaning activities and use of specific cleaning products among Spanish indoor cleaners. Scand. J. Work. Environ. Health 2001, 27, 76–81.
- Zhang, K.S.; Chen, H.Q.; Chen, Y.S.; Qiu, K.F.; Zheng, X.B.; Li, G.C.; Yang, H.D.; Wen, C.J. Bisphenol A stimulates human lung cancer cell migration via upregulation of matrix metalloproteinases by GPER/EGFR/ERK1/2 signal pathway. Biomed. Pharmacother. 2014, 68, 1037–1043.
- Jaakkola, J.J.; Knight, T.L. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: A systematic review and meta-analysis. Environ. Health Perspect. 2008, 116, 845–853.
- Hsu, N.Y.; Lee, C.C.; Wang, J.Y.; Li, Y.C.; Chang, H.W.; Chen, C.Y.; Bornehag, C.G.; Wu, P.C.; Sundell, J.; Su, H.J. Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air 2012, 22, 186–199.
- Kim, Y.M.; Kim, J.; Cheong, H.K. Exposure to phthalates aggravates pulmonary function and airway inflammation in asthmatic children. PLoS ONE 2018, 13, e0208553.
- Kim, J.H.; Bae, S.L.; Kiyoung, S.J.; Hong, Y. Exposure to Bisphenol A and Phthalates Affects Lung Function and Oxidative Stress in the Elderly. Epidemiology 2009, 20, S154.
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007, 3, 186–191.
- Yegambaram, M.; Manivannan, B.; Beach, T.G.; Halden, R.U. Role of environmental contaminants in the etiology of Alzheimer’s disease: A review. Curr. Alzheimer. Res. 2015, 12, 116–146.
- Kodavanti, P.R. Cell signaling and neurotoxicity: Protein kinase C in vitro and in vivo. Methods Mol. Biol. 2011, 758, 307–319.
- Zaganas, I.; Kapetanaki, S.; Mastorodemos, V.; Kanavouras, K.; Colosio, C.; Wilks, M.F.; Tsatsakis, A.M. Linking pesticide exposure and dementia What is the evidence? Toxicology 2013, 307, 3–11.
- Steenland, K.; Hein, M.J.; Cassinelli, R.T.; Prince, M.M.; Nilsen, N.B.; Whelan, E.A.; Waters, M.A.; Ruder, A.M.; Schnorr, T.M. Polychlorinated Biphenyls and Neurodegenerative Disease Mortality in an Occupational Cohort. Epidemiology 2006, 17, 8–13.
- Freire, C.; Koifman, S. Pesticide exposure and Parkinson’s disease: Epidemiological evidence of association. Neurotoxicology 2012, 33, 947–971.
- Parrón, T.; Requena, M.; Hernández, A.F.; Alarcón, R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol. Appl. Pharmacol. 2011, 256, 379–385.
- Hayden, K.M.; Norton, M.C.; Darcey, D.; Ostbye, T.; Zandi, P.P.; Breitner, J.C.; Welsh-Bohmer, K.A. Occupational exposure to pesticides increases the risk of incident AD: The Cache County study. Neurology 2010, 74, 1524–1530.
- Chalubinski, M.; Kowalski, M.L. Endocrine disrupters—Potential modulators of the immune system and allergic response. Allergy 2006, 61, 1326–1335.
- Robinson, L.; Miller, R. The impact of bisphenol A and phthalates on allergy, asthma, and immune function: A review of latest findings. Curr. Environ. Health Rep. 2015, 2, 379–387.
- Dewailly, E.; Ayotte, P.; Bruneau, S.; Gingras, S.; Belles-Isles, M.; Roy, R. Susceptibility to infections and immune status in Inuit infants exposed to organochlorines. Environ. Health Perspect. 2000, 108, 205–211.
- Heilmann, C.; Grandjean, P.; Weihe, P.; Nielsen, F.; Budtz-Jørgensen, E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006, 3, e311.
- Clayton, E.M.; Todd, M.; Dowd, J.B.; Aiello, A.E. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environ. Health Perspect. 2011, 119, 390–396.
- Hertz-Picciotto, I.; Park, H.Y.; Dostal, M.; Kocan, A.; Trnovec, Y. Pre-natal exposure to persistent and non-persistent organic compounds and effects on immune system development. Basic Clin. Pharmacol. Toxicol. 2008, 102, 146–154.
- Noakes, P.S.; Taylor, P.; Wilkinson, S.; Prescott, S.L. The relationship between persistent organic pollutants in maternal and neonatal tissues and immune responses to allergens: A novel expoloratory study. Chemosphere 2006, 63, 1304–1311.
- Horváthová, M.; Jahnová, E.; Palkovičová, L.; Trnovec, T.; Hertz-Picciotto, I. Dynamics of lymphocyte subsets in children living in an area polluted by polychlorinated biphenyls. J. Immunotoxicol. 2011, 8, 333–345.
- Zahra, A.; Sisu, C.; Silva, E.; De Aguiar Greca, S.C.; Randeva, H.S.; Chatha, K.; Kyrou, I.; Karteris, E. Is There a Link between Bisphenol A (BPA), a Key Endocrine Disruptor, and the Risk for SARS-CoV-2 Infection and Severe COVID-19? J. Clin. Med. 2020, 9, 3296.
- Kostoff, R.N.; Briggs, M.B.; Porter, A.L.; Hernández, A.F.; Abdollahi, M.; Aschner, M.; Tsatsakis, A. The under-reported role of toxic substance exposures in the COVID-19 pandemic. Food Chem. Toxicol. 2020, 145, 111687.
- Tsatsakis, A.; Petrakis, D.; Nikolouzakis, T.K.; Docea, A.O.; Calina, D.; Vinceti, M.; Goumenou, M.; Kostoff, R.N.; Mamoulakis, C.; Aschner, M.; et al. COVID-19, an opportunity to reevaluate the correlation between long-term effects of anthropogenic pollutants on viral epidemic/pandemic events and prevalence. Food Chem. Toxicol. 2020, 141, 111418.
- Wu, Q.; Coumoul, X.; Grandjean, P.; Barouki, R.; Audouze, K. Endocrine disrupting chemicals and COVID-19 relationships: A computational systems biology approach. Environ. Int. 2020.
- Ji, J.; Song, L.; Wang, J.; Yang, Z.; Yan, H.; Li, T.; Yu, L.; Jian, L.; Jiang, F.; Li, J.; et al. Association between urinary per- and poly-fluoroalkyl substances and COVID-19 susceptibility. Environ. Int. 2021, 153, 106524.