Infection with Leishmania parasites can lead to severe disease in humans and dogs, which act as a reservoir in zoonotic transmission. The identification of additional animal species recognised zoonotic reservoirs is of great importance to leishmaniasis control programs. In addition, Leishmanis spp. can cause considerable disease in animals other than dogs.
- infection
- Leishmania
- leishmaniosis
1. Introduction
The parasitic disease leishmaniasis is caused by vector-borne protozoa of the genus Leishmania and transmitted via infected female sand flies (Phlebotomus and Lutzomyia) [1]. The disease is prevalent in tropical and subtropical countries around the world. As a category 1 emerging and uncontrolled disease, leishmaniasis is considered a severely neglected disease and intensified research programs to improve vector control, diagnostics and the therapeutic arsenal to contain further incidence and morbidity are needed [1]. An infection with Leishmania parasites in humans can give rise to three main clinical manifestations, depending on infecting Leishmania spp. and several host factors, including immune-inflammatory processes [2]. The first is localized cutaneous leishmaniasis (CL) with single to multiple skin ulcers, satellite lesions or nodular lymphangitis [3]. The second is CL with mucosal involvement and the third systemic visceral leishmaniasis (VL) without involvement of the skin and affecting internal organs, such as liver, spleen and bone marrow, which is lethal if not appropriately treated [1,2]. Vector-borne transmission of leishmaniasis to humans can either be anthroponotic (from human to human) or zoonotic (from a non-human vertebrate reservoir to humans) [4]. The identification of the zoonotic reservoirs (i.e., animal populations which may be sources of Leishmania spp.) is of great importance to leishmaniasis control programs, in particular to design adequate interventions. In addition, Leishmania spp. can also cause considerable disease in animals. In the case of non-human animals, the term leishmaniosis is used to refer to the disease [5].
Dogs are considered to be the main or primary domestic reservoir for human infection in settings where zoonotic leishmaniosis occurs [6]. In fact, a significant proportion of infected dogs remain clinically healthy thanks to an adequate cell-mediated immune response, but these subclinically infected animals can act as carriers of Leishmania spp. and are capable of transmitting parasites to the sand fly vectors [7]. However, a variable time after infection, dogs may develop a systemic chronic disease with a broad spectrum of severity and clinical signs due to the dissemination of the parasite in the skin and internal organs and due to the development of immune-mediated pathology. Prognosis can be poor in dogs even when they are treated and chronic kidney disease is its most important determinant [8].
2. New Insights into the Future of Epidemiological Aspects of Animal Leishmaniosis in Europe
Apart from infectiousness to sand flies, the reservoir role and the risk to human and animal health, in particular to pets, arises from other important requirements not usually met by wild animals. For instance, in most cases there is no close contact between the wild animals and humans or pets and, as sand flies do not travel long distances [82,163], it is not likely that a lot of transmission will occur. However, as the example of human leishmaniasis outbreak in Madrid has shown, when there is close contact between humans (entering the niche, in this case city parks) and animals (in this case hares), and when the appropriate vectors are present, the high population density of a wild reservoir is able to sustain a human disease outbreak [108,128,134,164].
The situation might be different when there is more direct contact between humans and infected animals, in particular in case of domestic or farm animals. The present review has shown that there is not much evidence that livestock (cattle, sheep and goats) are infected with L. infantum. In contrast, based on the finding in this review, there is reason for concern that equids, and in particular horses (Figure 1), may be a more potential reservoir as increasing numbers of infections, with overt diseases, are being reported [150].
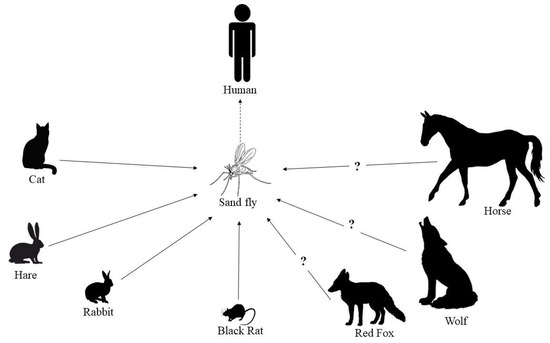
The feline population remains, in addition to dogs, the most important group to be affected by leishmaniosis. As reported in this review, subclinical and chronic feline infections are common in regions endemic for CanL [48,49,50]. Particularly where most dogs are well-protected against sand fly bites and often also by vaccination, cats may play a significant epidemiological role because of their high numbers and the limited use of ectoparasiticides effective in cats against sand fly bites. In fact, in some endemic areas, both pet and unowned cats outnumber dogs [165]. The significance of cats as a reservoir of Leishmania spp. and not simply as an accidental host is gaining more and more ground [166]. The recent systematic review by Asfaram et al. [167] further underpinned that cats act as primary and/or secondary reservoir hosts in the transmission of Leishmania spp. to humans and also to dogs, through sand flies, at least in endemic foci. This re-emphasises the recommendations for further research to close the knowledge gap in FeL and to gather more evidence-based information on the epidemiology, transmission and management of this disease [40]. Clinical illness due to L. infantum in cats is less frequent than in dogs, and though it is currently better recognized by feline practitioners than in the past, it is still underestimated and requires more veterinary attention [40,41,166]. Similarities with CanL exist and cats with L. infantum-associated clinical disease have high blood parasitaemia, low to very high antibody levels and hyperglobulinaemia; however, consolidated evidence-based knowledge about the disease associated with L. infantum infection in cats is limited, including risk factors, the role of innate and adaptive immune response in the pathogenesis and prognosis, and the more appropriate management of clinical cases.
The red fox is the most abundant wild carnivore in Europe, with relatively high population densities, and can be considered a species that connects the wild, rural and urban environments [168]. Red foxes have displayed high prevalence values and may serve as a secondary reservoir of L. infantum, but it is still necessary to elucidate whether they can be infectious to sand flies [11,36]. Several reports are also present in the literature about infected wolves, but the role of this wild species in the epidemiological cycle of L. infantum is still unclear (Figure 1).
The geographical spread of animal Leishmania infections is mainly confined to the more southern parts of Europe, in particular Greece, Italy, Spain and Portugal, where the Mediterranean climatological conditions for sand flies are probably more favourable. Few cases are reported from more temperate countries such as Germany and Switzerland [94,143]. The Nordic countries have so far not reported cases of animal infections with Leishmania in hosts other than dogs. Global warming may eventually change this and it is therefore important to become vigilant [9]. Additionally, travelling and rehoming of cats [94] or movements of wild animals between zoological parks can give rise to occasional clinical cases in non-endemic areas. Nowadays, it is not known whether non-vectorial transmission of L. infantum occurs in hosts other than dogs and humans. If this was the case, autochthonous foci could be observed out of endemic regions as is seen in dogs [169].
This entry is adapted from the peer-reviewed paper 10.3390/pathogens10030307
