Protein phosphorylation is a necessary mechanism to drive numerous cellular processes such as cell division, migration, differentiation and programmed cell death. This process is regulated by many enzymes, including cyclin-dependent kinases (CDKs) which phosphorylate proteins on their serine and threonine amino acid residues. The 20 members of CDK family known to this day regulate the cell cycle, transcription and splicing.
- cyclin-dependent kinase inhibitors
- cancer
- cell cycle
- CDKs
- CDK inhibitors
1. Cyclin-Dependent Kinases (CDKs)
Protein phosphorylation is a necessary mechanism to drive numerous cellular processes such as cell division, migration, differentiation and programmed cell death. This process is regulated by many enzymes, including cyclin-dependent kinases (CDKs) which phosphorylate proteins on their serine and threonine amino acid residues. The 20 members of CDK family known to this day regulate the cell cycle, transcription and splicing [1]. A number of kinase inhibitors are emerging every day as potential small molecule drugs, with some of them already being approved by the United States Food and Drug Administration (FDA). Moreover, these already approved kinase targeting drugs now account for more than a quarter of all available drugs [2]. In relation to CDK inhibitors, drugs such as Palbociclib 1, Ribociclib 2 and Abemaciclib 3, have been approved for ER+/HER2- advanced breast cancer treatment [3]. Until recently, the focus of the research was aimed at the highly conserved ATP binding sites of each CDK kinase. Hence, the development of CDK inhibitors has been extremely challenging due to the difficulty of obtaining sufficient selectivity with typical ATP-mimetic compounds. The greatest number of reported compounds has been identified to target the ATP binding pocket. Most recent studies suggest that inhibitors targeting hydrophobic pockets outside the ATP binding site may provide an opportunity for rational target selectivity [4]. Figure 1 illustrates the typical protein structure of the CDK enzyme. The diagram depicts the structural features of a typical kinase domain. Specifically highlighted are the binding pockets of different types of inhibitors, as well as the activation loop.
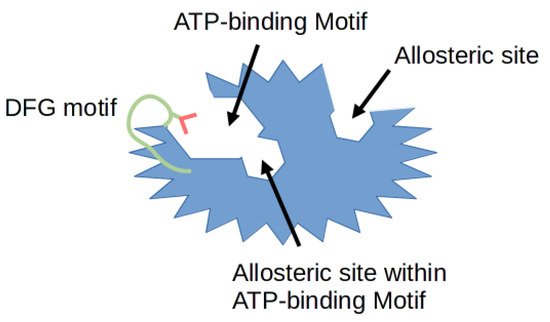
Figure 1. Schematic representation of different types of binding pockets. The protein kinase is shown in blue, with the Asp-Phe-Gly (DFG) motif in green. Red color denotes the aspartate amino acid residue of the DFG motif. The particular regions where different types of inhibitors bind are described below, the allosteric pocket is only a visualization and its place can be anywhere outside the ATP binding site.
2. Cyclin-Dependent Kinase (CDK) Inhibitors in Drug Development
CDK family is known to regulating the cell cycle, transcription and splicing. Deregulation of any of the stages of the cell cycle or transcription leads to apoptosis but, if uncorrected, it can result in a series of diseases such as cancer or neurodegenerative diseases [1][5][6][7].
Within the last 20 years important advances have been achieved in the development of effective strategies to inhibit CDK kinases. Access of the substrate to the active site of CDK kinase is regulated by the activation loop (A-loop) which is very flexible. The A-loop contains between 20–30 amino acids marked by the conserved Asp-Phe-Gly (DFG) tripeptide motif at the proximal end. Phosphorylation of the activation loop activates the kinase. In this state, the DFG sequence fits snugly into a hydrophobic back pocket adjacent to the ATP binding site. Conversely, in the inactive state the DFG motif swings outwards by partially blocking both the ATP and substrate binding pockets [8].
To date, six types of small molecule kinase inhibitors have been defined by the pharmaceutical industry based on their biochemical mechanisms of action (Figure 2). Type I inhibitors interact directly with the ATP binding site and react with the active form of the kinase which is in the DFG-in state and with a phosphorylated activation loop (activation segment). These inhibitors mimic the hydrogen bonds created between the adenine ring of the ATP and the hinge region of the enzyme. Type II inhibitors interact with a DFG-out catalytically inactive conformation of the enzyme and, like type I inhibitors, explore the hinge region and the adenine binding pocket. Type III inhibitors are non-competitive with ATP as they bind to the hydrophobic pocket next to the ATP-binding site, while type IV inhibitors bind away from the ATP binding pocket. Both, type III and IV inhibitors are allosteric in nature [8]. Type V inhibitors interact with two separate regions of the protein kinase domain. This group of inhibitors has been classified as bi-substrate inhibitors. These five classes of inhibitors interact reversibly, while type VI inhibitors form a covalent bond with their target kinase (Figure 2) [9].
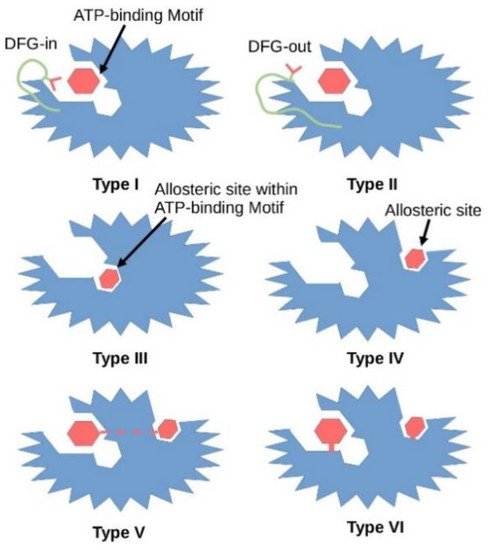
Figure 2. Graphical illustration of different types of kinase inhibitors and their mode of action. Dark red hexagon represents an inhibitor. The protein kinase is shown in blue, the DFG motif in green, the aspartate amino acid residue of the DFG motif in red. In 2015 Wu demonstrated that co-crystal structure of 3-phosphoinositide-dependent protein kinase 1 (PDPK1, PDK1) with ATP showed that type I inhibitors interact with the active conformation of the enzyme where the aspartate residue of the DFG motif points into the ATP binding pocket, while type II inhibitors stabilize the inactive conformation of the enzyme where the aspartate residue faces outward of the binding site (PDB entry: 4RRV). Type III inhibitors interact with the allosteric site within the ATP binding pocket. Type IV inhibitors interact with the allosteric site. However, the allosteric pocket is only a visualization and its place can be anywhere outside the ATP binding site. Type V inhibitors interact with both the allosteric and ATP binding pockets. Type VI inhibitors form covalent bonds with either the ATP binding pocket or the allosteric pocket.
3. Type I Inhibitors
Many heterocyclic compounds can mimic the hydrogen binding motif of adenine, therefore many type I inhibitors have been discovered. As mentioned above, these compounds interact with the ATP-site of the kinase in its active (DFG-in) conformation and with phosphorylated polypeptide region (activation segment) which lies outside the active site pocket. First generation of structurally diverse ATP competitive small molecule type I CDK inhibitors, produced in the late 1990s and early 2000s, have entered clinical trials to treat numerous solid tumors and hematopoietic malignances. Among the list of compounds that have been synthesized as CDK inhibitors, Flavopiridol (Alvocidib) 4 (Figure 3), a flavonoid derived from an indigenous plant from India, is active against CDK1, CDK2, CDK4, CDK6, and CDK9 with IC50 values in the 20–100 nM range (Table 1) [10][11][12][13]. Flavopiridol can inhibit cell cycle progression in G1 as well as G2 phase due to inhibition of CDK2/4 and CDK1 activity, respectively. Early clinical trials proved ineffective because of unsatisfactory efficacy and high toxicity [14][15]. However, later studies confirmed its clinical efficacy in hematological malignancies, and it was granted orphan drug designation for the treatment of patients with acute myeloid leukemia (Table 2) [16].
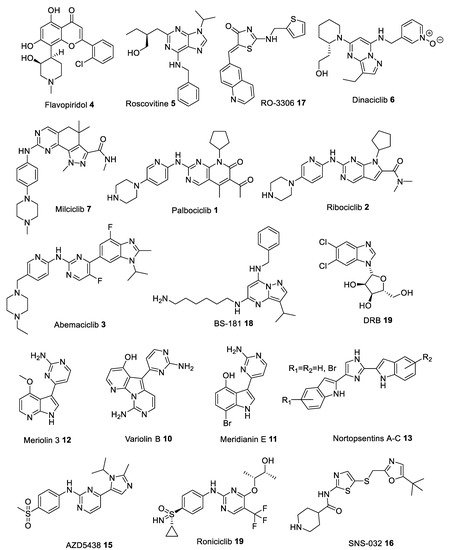
Figure 3. Chemical structures of some of the most studied type I cyclin-dependent kinase (CDK) inhibitors.
Roscovitine (Seliciclib) 5 (Figure 3), one of the best known CDK inhibitors, is active against CDK2, CDK5, CDK7 and CDK9 (Table 1). This compound is, by far, the most effective inhibitor of CDK5/p25 (IC50 = 160 nM [17]), as shown by numerous studies using this compound as a potential drug against cancer, neurodegenerative or viral diseases, inflammation, polycystic kidney disease (PKD) and glomerulonephritis (Table 2) [18][19][20][21][22]. However, despite many successful preclinical studies, results from several clinical trials are not that promising [23].
Another compound is Dinaciclib 6 (Figure 3), which proved to be a very effective small molecule inhibitor against CDK5 (IC50 = 1 nM [24]) (Table 1). Preclinical studies have shown that Dinaciclib is effective against solid tumors and chronic lymphocytic leukemia (CLL), without adversely affecting T-lymphocytes and their numbers (Table 2) [25].
Moreover, Milciclib 7 (Figure 3), an orally bioavailable inhibitor of cyclin-dependent kinases (CDKs) and several other protein kinases responsible for controlling cell growth and replication, has recently obtained the orphan drug designation for thymic carcinoma. It is currently under investigation as a potential drug target for treatment of glioma and hepatocellular carcinoma (HCC) (Table 2) [26][27]. It inhibits CDK2 with IC50 of 45 nM and exhibits submicromolar activity against other CDKs including CDK1, CDK4 and CDK5 resulting in a block in the G1 (gap) phase of the cell cycle (Table 1) [28]. Furthermore, Milciclib was found to reduce levels of microRNAs, miR-221 and miR-222, which promote the formation of blood supply (angiogenesis) in cancer tumors [29].
And finally, Palbociclib 1 and Ribociclib 2 (Figure 3), novel CDK4/6 inhibitors, were approved as effective drugs against HR+/HER2- metastatic breast cancer (Table 2) [30][31]. They selectively inhibit CDK4/6 (Table 1), thereby inhibiting retinoblastoma (Rb) protein phosphorylation early in the G1 phase leading to cell cycle arrest, causing defects in DNA replication and efficiently suppress cancer cell proliferation. Most recent data show that both drugs demonstrate a synergistic effect when combined with other drugs, for example Palbociclib and aromatase inhibitor Letrozole [32], Ribociclib and either anaplastic lymphoma kinase (ALK) inhibitor or the mitogen-activated protein kinase kinase (MAP2K, MEK) inhibitor Trametinib [33]. Moreover, utilizing this approach leads to a significant reduction in the development of resistance during prolonged treatment courses [31].
In addition, Tamoxifen 8 has been found to be effective against breast cancer. It reduces CDK5 activity by interacting with p25 and p35, thus preventing CDK5 activation. Tamoxifen can also lower Tau protein phosphorylation, which may suggest that tamoxifen could be used against Alzheimer’s disease [34].
Yet another inhibitor, 5,6-dichlorobenzimidazone-1-β-D-ribofuranoside (DRB) 9 (Figure 3) possesses high selectivity against CDK9, with nearly 25-fold difference in potency over CDK2 and CDK7 (Table 1) [35]. In HeLa cells, DRB (75 μM) inhibited 60-75% of nuclear heterogeneous RNA (hnRNA) synthesis. DRB inhibited a HeLa protein kinase which phosphorylated an RNA polymerase II-derived peptide [36]. DRB can also inhibit HIV transcription (IC50 = ~4 μM) by targeting elongation enhanced by the HIV-encoded transactivator Tat (Table 2) [37].
Table 1. Kinase inhibitory activities of type I CDK inhibitors.
| Inhibitor | Kinase IC50 [nM] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CDK1/B | CDK2/A | CDK2/E | CDK4/D | CDK5/p25 | CDK6/D | CDK7/H | CDK8/C | CDK9/T1 | |
| Flavopiridol 4 [38][39] | 30 | 100 | 100 | 20–40 | - | 60 | 110 | - | 20 |
| Roscovitine 5 [40] | 650 | 700 | 700 | >100,000 | 160 | >100,000 | 460 | >100,000 | 600 |
| RO-3306 17 [41] | 35 | - | 340 | >2000 | - | - | - | - | - |
| Dinaciclib 6 [42] | 3 | 1 | 1 | 100 | 1 | - | - | - | 4 |
| Milciclib 7 [28] | 398 | 45 | 363 | 160 | 265 | - | 150 | - | - |
| Palbociclib 1 [43] | >10,000 | >10,000 | >10,000 | 11 | >10,000 | 15 | - | - | - |
| Ribociclib 2 [44] | 113,000 | 76,000 | 76,000 | 10 | 43,900 | 39 | - | - | - |
| Abemaciclib 3 [45] | 1627 | - | 504 | 2 | 355 | 10 | 3910 | - | 57 |
| BS-181 18 [46] | 8100 | 730 | 880 | 33,000 | 3000 | 47,000 | 21 | - | 4200 |
| DRB 9 [47] | 17,000 | - | >10,000 | >10,000 | - | - | >10,000 | >10,000 | 340 |
| Meriolin 3 12 [48] | 170 | 11 | - | >100,000 | 170 | >100,000 | >100,000 | - | 6 |
| Variolin B 10 [49] | 60 | 80 | - | >10,000 | 90 | >10,000 | >1000 | - | 26 |
| Meridianin E 11 [50] | 180 | 800 | 1800 | 3000 | 150 | - | - | - | 18 |
| Nortopsentins 13 [51] | 310–900 | - | - | - | - | - | - | - | - |
| AZD5438 15 [52] | 16 | 45 | 6 | 449 | 14 | 21 | 821 | - | 20 |
| Roniciclib 19 [53] | 7 | - | 9 | 11 | - | - | 25 | - | 5 |
| SNS-032 16 [54] | 480 | 38 | 48 | 925 | 340 (CDK5/p35) |
- | 62 | - | 4 |
Table 2. Type I CDK inhibitors at different phases of clinical and pre-clinical studies. Trial information obtained from ClinicalTrials.gov as of January 2021.
| Inhibitor | Main Targets | Condition or Disease | Phase | Status | Identifier |
|---|---|---|---|---|---|
| Flavopiridol 4 | CDK1, CDK2, CDK4, CDK6, CDK9 | Acute Myeloid Leukemia (AML) | on the market | “orphan drug” | - |
| Roscovitine 5 | CDK2, CDK7, CDK9 | Pituitary Cushing Disease | II | active | NCT02160730 NCT03774446 |
| Cystic Fibrosis | II | terminated | NCT02649751 | ||
| Advanced Solid Tumors | I | terminated | NCT00999401 | ||
| Lung Cancer | II | terminated | NCT00372073 | ||
| RO-3306 17 [41] | CDK1 | Acute Myeloid Leukemia (AML) | pre-clinical | - | - |
| Dinaciclib 6 | CDK1, CDK2, CDK5, CDK9 | Chronic Lymphocytic Leukemia (CLL) | on the market | “orphan drug” | - |
| Breast and Lung Cancers | II | terminated | NCT00732810 | ||
| Milciclib 7 | CDK1, CDK2, CDK4, CDK7 | Hepatocellular Carcinoma (HCC) | II | active | NCT03109886 |
| Thymic Carcinoma | II | terminated | NCT01301391 NCT01011439 |
||
| Palbociclib 1 | CDK4, CDK6 | HR+/HER2- Breast Cancer | on the market | used in combination with Letrozole | - |
| III | active, to be used with other drugs like Fulvestrant | NCT02692755 | |||
| Head and Neck, Brain, Colon, and other Solid Cancers | II | active, to be used alone and in combination with different drugs | NCT02255461 NCT03446157 NCT02896335 NCT03965845 |
||
| Ribociclib 2 | CDK4, CDK6 | HR+/HER2- Breast Cancer | on the market | used in combination with Letrozole | - |
| III | active, to be used with other drugs like Fulvestrant | NCT02422615 NCT03439046 NCT03294694 |
|||
| Prostate, and other Solid Cancers | II | active, to be used alone and in combination with different drugs | NCT02555189 NCT01543698 NCT02934568 |
||
| Abemaciclib 3 | CDK4, CDK6 | HR+/HER2- Breast Cancer | on the market | used in combination with Fulvestrant | - |
| III | active, to be used with other drugs like Letrozole | NCT02763566 | |||
| Lung, Brain, Colon, and other Solid Cancers | II or III | active, to be used alone and in combination with different drugs | NCT04545710 NCT02152631 NCT03220646 NCT04616183 NCT03310879 |
||
| BS-181 18 [46] | CDK7 | Breast, Lung, Prostate and Colorectal Cancers | pre-clinical | - | - |
| DRB 9 [55] | CDK7, CDK8, CDK9 | HIV Transcription | pre-clinical | - | - |
| Meriolin 3 12 [48] | CDK1, CDK2, CDK5, CDK9 | Neuroblastoma, Glioma, Myeloma, Colon Cancer | pre-clinical | - | - |
| Variolin B 10 [56] | CDK1, CDK2, CDK5, CDK9 | Murine Leukemia | pre-clinical | - | - |
| Meridianin E 11 [57] | CDK1, CDK5, CDK9 | Larynx Carcinoma, Myeloid Leukemia | pre-clinical | - | - |
| Nortopsentins 13 [58] | CDK1 | Malignant Pleural Mesothelioma (MPM) | pre-clinical | - | - |
| AZD5438 15 | CDK1, CDK2, CDK5, CDK6, CDK9 | Advanced Solid Malignancies | I | terminated | NCT00088790 |
| Roniciclib 19 | CDK1, CDK2, CDK4, CDK7, CDK9 | Lung and Advanced Solid Cancers | II | terminated | NCT02161419 NCT01573338 NCT02656849 |
| SNS-032 16 | CDK2, CDK7, CDK9 | Chronic Lymphocytic Leukemia and other Solid Cancers | I | terminated | NCT00446342 NCT00292864 |
Novel alkaloids, acting as CDK inhibitors, were also found in some marine organisms. Variolins, 7-azaindole based alkaloids isolated from the antarctic sponge Kirkpatrickia variolosa [59][60], showed in vitro activity against a murine (P388) leukemia cell line with submicromolar potencies by preventing cell proliferation, and inducing apoptosis (Table 2) [56][59]. Variolin B 10 (Figure 3), in particular, was found to inhibit CDK1 and CDK2 kinases, in the micromolar concentration range (Table 1) [61]. Meridianins A-G, a family of 3-(2-aminopyrimidine)indoles, which originate from the ascidian Aplidium meridianum, were demonstrated to inhibit several protein kinases, especially Meridianin E 11 (Figure 3), which can selectively inhibit CDK1 and CDK5 in the low micromolar range (Table 1) [62]. Based on the latter two compounds, Meriolins 12, a new class of inhibitors, have been designed. These new derivatives have been reported to strongly inhibit various protein kinases, especially CDK1, CDK2, CDK4 and CDK9 (Table 1) [48]. Most recent analysis provides a high potential of Meriolins in the treatment of cancer and noncancer pathologies such as polycystic kidney disease, neurodegenerative diseases, stroke, chronic inflammation, and bipolar disorders (Table 2) [48]. Nortopsentins A-C 13 (Figure 3), antifungal 1,4-bisindolylimidazole marine alkaloids, having an imidazole as a spacer between the two indole units, isolated from the Caribbean deep sea Spongosorites ruetzleri, displayed in vitro cytotoxicity against P388 leukemia cells (IC50 4.5–20.7 µM). Analogues in which the imidazole ring of the alkaloid was replaced by other five or six membered heterocycles were able to inhibit the activity of the cyclin-dependent kinase 1 (CDK1) with submicromolar IC50 values (in particular 3-[(2-indolyl)-5-phenyl]-pyridines, phenyl-thiazolyl-7-azaindoles, indolyl-thiazolyl-4-azaindole and indolyl-thiazolyl-7-azaindole derivatives) (Table 1) [51]. Preliminary results indicate, that Nortopsentins, and their analogues, were active against malignant pleural mesothelioma (MPM), a very aggressive human malignancy poorly responsive to currently available therapies (Table 2) [58].
Recent development has enabled combinatorial treatment regimens which can demonstrate synergistic anticancer mechanisms. For instance, THZ1 14 (Figure 7) a covalent CDK7 inhibitor, was found to selectively downregulate CDK7-mediated phosphorylation of RNA polymerase II, indicative of transcriptional inhibition. Further investigations revealed that the survival of triple negative breast cancer (TNBC) cells relied heavily on the B-cell lymphoma 2 (BCL-2)/B-cell lymphoma-extra large (BCL-XL) signaling axes in cells. Thus, combining the CDK7 inhibitor THZ1 with the BCL-2/BCL-XL inhibitors (ABT-263/ABT199) offer a preclinical proof to significantly improve the poor prognosis in TNBC [63].
However, the complexity of CDK biology and the undesired toxicity related to the off-target effects of the existing pan-CDK inhibitors, led to decisions by several pharmaceutical companies to discontinue the development of many potential anti-cancer agents, exampled with AZD5438 15, Roniciclib, SNS-032 16, RO-3306 17, BS-181 18 and Roniciclib 19 (Figure 3) (Table 1 and Table 2) [64][65][66]. Therefore, new classes of more selective CDK inhibitors, with strong potential to deliver a meaningful therapeutic impact, were needed.
One of those compounds is CDK5 inhibitory protein (CIP), a small protein which contribute to nerve cells’ degeneration. CIP specifically blocks the hyperactivated state of CDK5 only when it is linked to p25/p29, while allowing normal activation of CDK5 by p35/p39. The selective inhibition of p25/CDK5 hyperactivation in vivo, through overexpression of CIP, reduced neurodegeneration and improved cognitive function of transgenic mice, without affecting normal neurodevelopment [67]. These findings suggest that CIP could possibly be used to selectively inhibit the p25/CDK5 hyperactivation as a potential therapeutic target to treat certain cancers caused by aberrant CDK5 activation.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22062806
References
- Pavletich, N.P. Mechanisms of cyclin-dependent kinase regulation: Structures of cdks, their cyclin activators, and cip and INK4 inhibitors. J. Mol. Biol. 1999, 287, 821–828.
- Cohen, P. Protein kinases—The major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309–315.
- Marra, A.; Curigliano, G. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer 2019, 5, 27.
- Wu, P.; Clausen, M.H.; Nielsen, T.E. Allosteric small-molecule kinase inhibitors. Pharmacol. Ther. 2015, 156, 59–68.
- Morgan, D.O. The Cell Cycle: Principles of Control, 1st ed.; New Science Press: London, UK, 2007.
- Patrick, G.N.; Zukerberg, L.R.; Nikolic, M.; De La Monte, S.; Dikkes, P.; Tsai, L.-H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nat. Cell Biol. 1999, 402, 615–622.
- Osuga, H.; Osuga, S.; Wang, F.; Fetni, R.; Hogan, M.J.; Slack, R.S.; Hakim, A.M.; Ikeda, J.-E.; Park, D.S. Cyclin-dependent kinases as a therapeutic target for stroke. Proc. Natl. Acad. Sci. USA 2000, 97, 10254–10259.
- Zhao, Z.; Wu, H.; Wang, L.; Liu, Y.; Knapp, S.; Liu, Q.; Gray, N.S. Exploration of Type II Binding Mode: A Privileged Approach for Kinase Inhibitor Focused Drug Discovery? ACS Chem. Biol. 2014, 9, 1230–1241.
- Rabiller, M.; Getlik, M.; Klüter, S.; Richters, A.; Tückmantel, S.; Simard, J.R.; Rauh, D. Proteus in the World of Proteins: Conformational Changes in Protein Kinases. Arch. Pharm. 2010, 343, 193–206.
- Baumli, S.; Lolli, G.; Lowe, E.D.; Troiani, S.; Rusconi, L.; Bullock, A.N.; Debreczeni, J.É.; Knapp, S.; Johnson, L.N. The structure of P-TEFb (CDK9/cyclin T1), its complex with flavopiridol and regulation by phosphorylation. EMBO J. 2008, 27, 1907–1918.
- Chao, S.-H.; Fujinaga, K.; Marion, J.E.; Taube, R.; Sausville, E.A.; Senderowicz, A.M.; Peterlin, B.M.; Price, D.H. Flavopiridol Inhibits P-TEFb and Blocks HIV-1 Replication. J. Biol. Chem. 2000, 275, 28345–28348.
- A Carlson, B.; Dubay, M.M.; A Sausville, E.; Brizuela, L.; Worland, P.J. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996, 56, 2973–2978.
- De Azevedo, W.F.; Mueller-Dieckmann, H.J.; Schulzegahmen, U.; Worland, P.J.; A Sausville, E.; Kim, S.H. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proc. Natl. Acad. Sci. USA 1996, 93, 2735–2740.
- E Kahn, M.; Senderowicz, A.; A Sausville, E.; E Barrett, K. Possible mechanisms of diarrheal side effects associated with the use of a novel chemotherapeutic agent, flavopiridol. Clin. Cancer Res. 2001, 7, 343.
- Stadler, W.M.; Vogelzang, N.J.; Amato, R.; Sosman, J.; Taber, D.; Liebowitz, D.; Vokes, E.E. Flavopiridol, A Novel Cyclin-Dependent Kinase Inhibitor, in Metastatic Renal Cancer: A University of Chicago Phase II Consortium Study. J. Clin. Oncol. 2000, 18, 371.
- Brown, J.R. Chronic Lymphocytic Leukemia: A Niche for Flavopiridol? Clin. Cancer Res. 2005, 11, 3971–3973.
- De Azevedo, W.F., Jr.; Gaspar, R.T.; Canduri, F.; Camera, J.C., Jr.; da Silveira, N.J.F.J.B. Molecular model of cyclin-dependent kinase 5 complexed with roscovitine. Biochem. Biophy. Res. Commun. 2002, 297, 1154–1158.
- Blachly, J.S.; Byrd, J.C. Emerging drug profile: Cyclin-dependent kinase inhibitors. Leuk. Lymphoma 2013, 54, 2133–2143.
- Pippin, J.W.; Qu, Q.; Meijer, L.; Shankland, S.J. Direct in vivo inhibition of the nuclear cell cycle cascade in experimental mesangial proliferative glomerulonephritis with Roscovitine, a novel cyclin-dependent kinase antagonist. J. Clin. Investig. 1997, 100, 2512–2520.
- Hoogendijk, A.J.; Roelofs, J.J.T.H.; Duitman, J.; Van Lieshout, M.H.P.; Blok, D.C.; Van Der Poll, T.; Wieland, C.W. R-roscovitine Reduces Lung Inflammation Induced by Lipoteichoic Acid and Streptococcus pneumoniae. Mol. Med. 2012, 18, 1086–1095.
- Schang, L.M.; Bantly, A.; Knockaert, M.; Shaheen, F.; Meijer, L.; Malim, M.H.; Gray, N.S.; Schaffer, P.A. Pharmacological Cyclin-Dependent Kinase Inhibitors Inhibit Replication of Wild-Type and Drug-Resistant Strains of Herpes Simplex Virus and Human Immunodeficiency Virus Type 1 by Targeting Cellular, Not Viral, Proteins. J. Virol. 2002, 76, 7874–7882.
- Patrick, C.; Crews, L.; Desplats, P.; Dumaop, W.; Rockenstein, E.; Achim, C.L.; Everall, I.P.; Masliah, E. Increased CDK5 Expression in HIV Encephalitis Contributes to Neurodegeneration via Tau Phosphorylation and Is Reversed with Roscovitine. Am. J. Pathol. 2011, 178, 1646–1661.
- Cicenas, J.; Kalyan, K.; Sorokinas, A.; Stankunas, E.; Levy, J.; Meskinyte, I.; Stankevicius, V.; Kaupinis, A.; Valius, M. Roscovitine in cancer and other diseases. Ann. Transl. Med. 2015, 3, 135.
- Parry, D.; Guzi, T.; Shanahan, F.; Davis, N.; Prabhavalkar, D.; Wiswell, D.; Seghezzi, W.; Paruch, K.; Dwyer, M.P.; Doll, R.; et al. Dinaciclib (SCH 727965), a Novel and Potent Cyclin-Dependent Kinase Inhibitor. Mol. Cancer Ther. 2010, 9, 2344–2353.
- Flynn, J.; Jones, J.; Johnson, A.J.; Andritsos, L.; Maddocks, K.; Jaglowski, S.; Hessler, J.; Grever, M.R.; Im, E.; Zhou, H.; et al. Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia 2015, 29, 1524–1529.
- Yakisich, J.S.; Vita, M.F.; Siden, Å.; Tasat, D.R.; Cruz, M. Strong inhibition of replicative DNA synthesis in the developing rat cerebral cortex and glioma cells by roscovitine. Investig. New Drugs 2009, 28, 299–305.
- Le Tourneau, C.; Faivre, S.; Laurence, V.; Delbaldo, C.; Vera, K.; Girre, V.; Chiao, J.; Armour, S.; Frame, S.; Green, S.R.; et al. Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur. J. Cancer 2010, 46, 3243–3250.
- Brasca, M.G.; Amboldi, N.; Ballinari, D.; Cameron, A.; Casale, E.; Cervi, G.; Colombo, M.; Colotta, F.; Croci, V.; D’Alessio, R.; et al. Identification of N,1,4,4-Tetramethyl-8-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-4,5-dihydro-1H-pyrazolo [4,3-h]quinazoline-3-carboxamide (PHA-848125), a Potent, Orally Available Cyclin Dependent Kinase Inhibitor. J. Med. Chem. 2009, 52, 5152–5163.
- Milciclib. Available online: (accessed on 20 January 2021).
- Rocca, A.; Farolfi, A.; Bravaccini, S.; Schirone, A.; Amadori, D. Palbociclib (PD 0332991): Targeting the cell cycle machinery in breast cancer. Expert Opin. Pharmacother. 2014, 15, 407–420.
- Samson, K. LEE011 CDK Inhibitor Showing Early Promise in Drug-Resistant Cancers. Oncol. Times 2014, 36, 39–40.
- Diéras, V.; Harbeck, N.; Joy, A.A.; Gelmon, K.; Ettl, J.; Verma, S.; Lu, D.R.; Gauthier, E.; Schnell, P.; Mori, A.; et al. Palbociclib with Letrozole in Postmenopausal Women with ER+/HER2− Advanced Breast Cancer: Hematologic Safety Analysis of the Randomized PALOMA-2 Trial. Oncologist 2019, 24, 1514.
- Sosman, J.A.; Kittaneh, M.; Lolkema, M.P.J.K.; Postow, M.A.; Schwartz, G.; Franklin, C.; Matano, A.; Bhansali, S.; Parasuraman, S.; Kim, K. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: Early encouraging clinical activity. J. Clin. Oncol. 2014, 32, 9009.
- Corbel, C.; Zhang, B.; Le Parc, A.; Baratte, B.; Colas, P.; Couturier, C.; Kosik, K.S.; Landrieu, I.; Le Tilly, V.; Bach, S. Tamoxifen Inhibits CDK5 Kinase Activity by Interacting with p35/p25 and Modulates the Pattern of Tau Phosphorylation. Chem. Biol. 2015, 22, 472–482.
- Maccallum, D.E. Seliciclib (CYC202, R-Roscovitine) Induces Cell Death in Multiple Myeloma Cells by Inhibition of RNA Polymerase II-Dependent Transcription and Down-regulation of Mcl-1. Cancer Res. 2005, 65, 5399–5407.
- Stevens, A.; Maupin, M.K. 5,6-Dichloro-1-β-D-ribofuranosylbenzimidazole inhibits a HeLa protein kinase that phosphorylates an RNA polymerase II-derived peptide. Biochem. Biophys. Res. Commun. 1989, 159, 508–515.
- Zhu, Y.; Pe’Ery, T.; Peng, J.; Ramanathan, Y.; Marshall, N.; Marshall, T.; Amendt, B.; Mathews, M.B.; Price, D.H. Transcription elongation factor P-TEFb is required for HIV-1 Tat transactivation in vitro. Genes Dev. 1997, 11, 2622–2632.
- Sedlacek, H. Mechanisms of action of flavopiridol. Crit. Rev. Oncol. 2001, 38, 139–170.
- Montagnoli, A.; Valsasina, B.; Croci, V.; Menichincheri, M.; Rainoldi, S.; Marchesi, V.; Tibolla, M.; Tenca, P.; Brotherton, D.; Albanese, C.; et al. A Cdc7 kinase inhibitor restricts initiation of DNA replication and has antitumor activity. Nat. Chem. Biol. 2008, 4, 357–365.
- Bach, S.; Knockaert, M.; Reinhardt, J.; Lozach, O.; Schmitt, S.; Baratte, B.; Koken, M.; Coburn, S.P.; Tang, L.; Jiang, T.; et al. Roscovitine Targets, Protein Kinases and Pyridoxal Kinase. J. Biol. Chem. 2005, 280, 31208–31219.
- Vassilev, L.T.; Tovar, C.; Chen, S.; Knezevic, D.; Zhao, X.; Sun, H.; Heimbrook, D.C.; Chen, L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. USA 2006, 103, 10660–10665.
- Zhang, D.; Mita, M.; Shapiro, G.I.; Poon, J.; Small, K.; Tzontcheva, A.; Kantesaria, B.; Zhu, Y.; Bannerji, R.; Statkevich, P. Effect of aprepitant on the pharmacokinetics of the cyclin-dependent kinase inhibitor dinaciclib in patients with advanced malignancies. Cancer Chemother. Pharmacol. 2012, 70, 891–898.
- Fry, D.W.; Harvey, P.J.; Keller, P.R.; Elliott, W.L.; Meade, M.; Trachet, E.; Albassam, M.; Zheng, X.; Leopold, W.R.; Pryer, N.K.; et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer 2004, 3, 1427.
- Tripathy, D.; Bardia, A.; Sellers, W.R. Ribociclib (LEE011): Mechanism of Action and Clinical Impact of This Selective Cyclin-Dependent Kinase 4/6 Inhibitor in Various Solid Tumors. Clin. Cancer Res. 2017, 23, 3251–3262.
- Gelbert, L.M.; Cai, S.; Lin, X.; Sanchez-Martinez, C.; Del Prado, M.; Lallena, M.J.; Torres, R.; Ajamie, R.T.; Wishart, G.N.; Flack, R.S.; et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: In-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig. New Drugs 2014, 32, 825–837.
- Ali, S.; Heathcote, D.A.; Kroll, S.H.B.; Jogalekar, A.S.; Scheiper, B.; Patel, H.; Brackow, J.; Siwicka, A.; Fuchter, M.J.; Periyasamy, M.; et al. The Development of a Selective Cyclin-Dependent Kinase Inhibitor That Shows Antitumor Activity. Cancer Res. 2009, 69, 6208–6215.
- Wang, S.; Fischer, P. Cyclin-dependent kinase 9: A key transcriptional regulator and potential drug target in oncology, virology and cardiology. Trends Pharmacol. Sci. 2008, 29, 302–313.
- Echalier, A.; Bettayeb, K.; Ferandin, Y.; Lozach, O.; Clément, M.; Valette, A.; Liger, F.; Marquet, B.; Morris, J.C.; Endicott, J.A.; et al. Meriolins (3-(Pyrimidin-4-yl)-7-azaindoles): Synthesis, Kinase Inhibitory Activity, Cellular Effects, and Structure of a CDK2/Cyclin A/Meriolin Complex†. J. Med. Chem. 2008, 51, 737–751.
- Bettayeb, K.; Tirado, O.M.; Marionneau-Lambot, S.; Ferandin, Y.; Lozach, O.; Morris, J.C.; Mateo-Lozano, S.; Drueckes, P.; Schächtele, C.; Kubbutat, M.H.; et al. Meriolins, a New Class of Cell Death–Inducing Kinase Inhibitors with Enhanced Selectivity for Cyclin-Dependent Kinases. Cancer Res. 2007, 67, 8325–8334.
- Sonawane, Y.A.; Taylor, M.A.; Napoleon, J.V.; Rana, S.; Contreras, J.I.; Natarajan, A. Cyclin Dependent Kinase 9 Inhibitors for Cancer Therapy. J. Med. Chem. 2016, 59, 8667–8684.
- Parrino, B.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Diana, P.; Cirrincione, G.; Carbone, A. Synthesis, antitumor activity and CDK1 inhibiton of new thiazole nortopsentin analogues. Eur. J. Med. Chem. 2017, 138, 371–383.
- Byth, K.F.; Thomas, A.; Hughes, G.; Forder, C.; McGregor, A.; Geh, C.; Oakes, S.; Green, C.; Walker, M.; Newcombe, N.; et al. AZD5438, a potent oral inhibitor of cyclin-dependent kinases 1, 2, and 9, leads to pharmacodynamic changes and potent antitumor effects in human tumor xenografts. Mol. Cancer Ther. 2009, 8, 1856–1866.
- Siemeister, G.; Lücking, U.; Wengner, A.M.; Lienau, P.; Steinke, W.; Schatz, C.; Mumberg, D.; Ziegelbauer, K. BAY 1000394, a Novel Cyclin-Dependent Kinase Inhibitor, with Potent Antitumor Activity in Mono- and in Combination Treatment upon Oral Application. Mol. Cancer Ther. 2012, 11, 2265–2273.
- Chen, R.; Wierda, W.G.; Chubb, S.; Hawtin, R.E.; Fox, J.A.; Keating, M.J.; Gandhi, V.; Plunkett, W. Mechanism of action of SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood 2009, 113, 4637–4645.
- Mousseau, G.; Mediouni, S.; Valente, S.T. Targeting HIV transcription: The quest for a functional cure. Curr. Top. Microbiol. Immunol. 2015, 389, 121–145.
- Walker, S.R.; Carter, E.J.; Huff, B.C.; Morris, J.C. Variolins and Related Alkaloids. Chem. Rev. 2009, 109, 3080–3098.
- Gompel, M.; Leost, M.; Joffe, E.B.D.K.; Puricelli, L.; Franco, L.H.; Palermo, J.; Meijer, L. Meridianins, a new family of protein kinase inhibitors isolated from the Ascidian Aplidium meridianum. Bioorganic Med. Chem. Lett. 2004, 14, 1703–1707.
- Carbone, A.; Pennati, M.; Parrino, B.; Lopergolo, A.; Barraja, P.; Montalbano, A.; Spanò, V.; Sbarra, S.; Doldi, V.; De Cesare, M.; et al. Novel 1H-Pyrrolo[2,3-b]pyridine Derivative Nortopsentin Analogues: Synthesis and Antitumor Activity in Peritoneal Mesothelioma Experimental Models. J. Med. Chem. 2013, 56, 7060–7072.
- Perry, N.B.; Ettouati, L.; Litaudon, M.; Blunt, J.W.; Munro, M.H.; Parkin, S.; Hope, H. Alkaloids from the antarctic sponge Kirkpatrickia varialosa. Tetrahedron 1994, 50, 3987–3992.
- Trimurtulu, G.; Faulkner, D.; Perry, N.B.; Ettouati, L.; Litaudon, M.; Blunt, J.W.; Munro, M.H.; Jameson, G.B. Alkaloids from the antarctic sponge Kirkpatrickia varialosa. Part 2: Variolin A and N(3′)-methyl tetrahydrovariolin B. Tetrahedron 1994, 50, 3993–4000.
- Simone, M.; Erba, E.; Damia, G.; Vikhanskaya, F.; Di Francesco, A.M.; Riccardi, R.; Bailly, C.; Cuevas, C.; Sousa-Faro, J.M.F.; D’Incalci, M. Variolin B and its derivate deoxy-variolin B: New marine natural compounds with cyclin-dependent kinase inhibitor activity. Eur. J. Cancer 2005, 41, 2366–2377.
- Bharate, S.B.; Yadav, R.R.; Battula, S.; Vishwakarma, R.A. Meridianins: Marine-derived potent kinase inhibitors. Mini-Reviews Med. Chem. 2012, 12, 618–631.
- Li, B.; Chonghaile, T.N.; Fan, Y.; Madden, S.F.; Klinger, R.; O’Connor, A.E.; Walsh, L.; O’Hurley, G.; Udupi, G.M.; Joseph, J.; et al. Therapeutic Rationale to Target Highly Expressed CDK7 Conferring Poor Outcomes in Triple-Negative Breast Cancer. Cancer Res. 2017, 77, 3834–3845.
- Walsby, E.; Lazenby, M.; Pepper, C.; Burnett, A.K. The cyclin-dependent kinase inhibitor SNS-032 has single agent activity in AML cells and is highly synergistic with cytarabine. Leukemia 2011, 25, 411–419.
- Reck, M.; Horn, L.; Novello, S.; Barlesi, F.; Albert, I.; Juhász, E.; Kowalski, D.; Robinet, G.; Cadranel, J.; Bidoli, P.; et al. Phase II Study of Roniciclib in Combination with Cisplatin/Etoposide or Carboplatin/Etoposide as First-Line Therapy in Patients with Extensive-Disease Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 701–711.
- Boss, D.; Schwartz, G.; Middleton, M.; Amakye, D.; Swaisland, H.; Midgley, R.; Ranson, M.; Danson, S.; Calvert, H.; Plummer, R.; et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of the oral cyclin-dependent kinase inhibitor AZD5438 when administered at intermittent and continuous dosing schedules in patients with advanced solid tumours. Ann. Oncol. 2010, 21, 884–894.
- Sundaram, J.R.; Poore, C.P.; Bin Sulaimee, N.H.; Pareek, T.; Asad, A.B.M.A.; Rajkumar, R.; Cheong, W.F.; Wenk, M.R.; Dawe, G.S.; Chuang, K.-H.; et al. Specific Inhibition of p25/Cdk5 Activity by the Cdk5 Inhibitory Peptide Reduces Neurodegeneration In Vivo. J. Neurosci. 2013, 33, 334–343.
