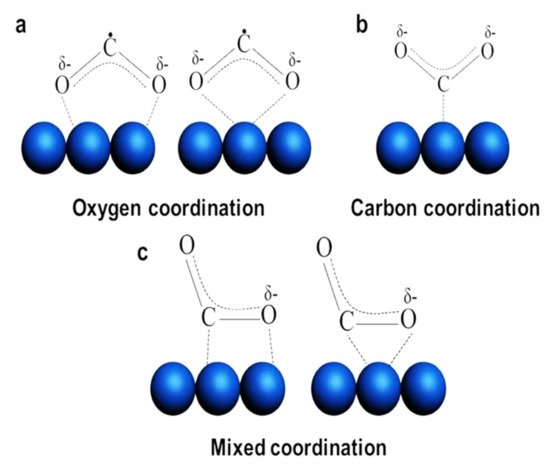
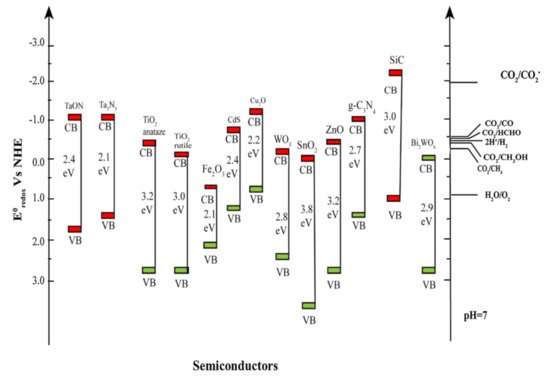
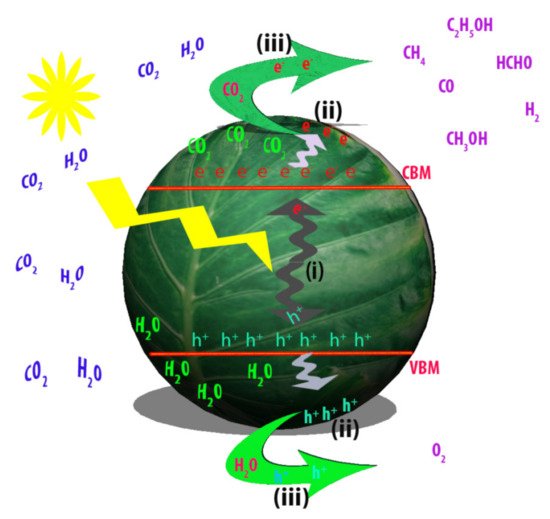
Applying low-dimensional structure to the photocatalytic system itself is a proven way for obtaining high CO2 conversion performance. However, further improvement is available by designing the structures or modifying the photocatalytic materials so that characteristics of photocatalysts or photocatalytic system are engineered. Here, we focus on these two major strategies for further enhancement of CO2 conversion by low-dimensional photocatalysts. summarizes the examples mentioned in this section.
Table 2. Summary of low-dimensional photocatalysts used in photocatalytic reduction of CO2 into solar fuels. NC: Nanocrystal, GQD: Graphene Quantum Dot, NF: Nanofiber, NS: Nanosheet, CNT: carbon nanotube, Mt: montmorillonite, m-CN: modified g-C3N4, NR: nanorod, NW: nanowire, CND: carbon nano dot, p-CN: protonated g-C3N4, PGCN: porous g-C3N4, TEOA: triethanolamine, bpy: bipyridine, C3N4: Melon-based polymeric carbon nitride, UTNS: ultrathin nanosheet, P-g-C3N4: Phosphorus doped g-C3N4.
3.1. Construction of Junction Formed by Low-Dimensional Structures
Of the diverse strategies to promote CO
2 conversion performance of photocatalysts, designing or formation of junctions in photocatalytic systems has seen some success by modifying optical and electrical properties through materials and interfaces [
63]. Junctions are constructed by coupling of two or more semiconductor materials or metal materials. The formation of a junction allows valuable properties from various materials to be more available in a single system. That helps more efficient light absorption, charge separation and transfer, or more stable performance. Since the photocatalytic systems with junctions have single or multiple interfaces, the engineering of the interfacial characteristics between the materials is essential for efficient photocatalyst performance. Especially, the interface characteristics can influence on carrier behaviors through bulk or at interfaces (ex. Shockley-Read-Hall recombination).
The formation of junctions implies the presence of an internal electric field in the nanomaterial. This internal electric field can contribute to enhanced carrier behaviors such as carrier separation and transfer for the photo-induced charge carriers and to, in the end, the performance of light-driven CO
2 conversion. The internal electric field can be induced by growth of a low-dimensional semiconductor on a low-dimensional semiconductor [
23,
107]. The combination of two or multiple low-dimensional materials could integrate the advantages of both single units and mitigate the shortcomings of single unit by the synergistic effect [
142].
One of the advantages of semiconductor QDs is the quantum size effect which is responsible for the optical properties of the photocatalyst. Apart from the acceleration of charge separation and transfer process, the contact between 0D semiconductor and 1D semiconductor provides the nanocomposite with an additional properties such as excessive electroactive sites, high surface area, and homogenous dispersion [
143]. 1D Bi
2S
3 nanotubes have outstanding ability to absorb visible and near infrared light. The tubular structure of Bi
2S
3 provides the photocatalytic reaction with more active sites than other morphologies [
58]. The remarkable optical and electronic properties of tungsten disulfide (WS
2) QDs can be realized due to the quantum confinement effect. WS
2 QDs can be also dispersed uniformly on the surface of Bi
2S
3 forming Bi–S channels to facilitate the charge carrier separation transfer process. The designed 0D/1D nanocomposite exhibited outstanding photocatalytic reduction of CO
2 into CH
3OH and C
2H
5OH of 38.2 µmol∙g
−1 and 27.8 µmol∙g
−1 after 4 h radiation, respectively. The improved photoreduction performance is related to the following features. Firstly, the 0D/1D nanocomposite provided combined optical and electrical properties of both WS
2 QDs and Bi
2S
3 nanotubes causing high visible and near infrared light absorption. Secondly, the enlarged surface area of the nanocomposite provided more active adsorptions site for CO
2. Thirdly, the low resistive QDs–NTs interface due to the Bi–S bonds plays a critical role for accelerated charge carrier separation [
58]. CsPbBr
3 is widely used in the photocatalytic reactions but it suffers from the high rate of recombination during the interface transfer due to the strong reductive ability of electrons [
64]. Hence, the suppression of undesired electron loss throughout the transfer process at the interface is critical factor for efficient utilization of CsPbBr
3. Li et al. fabricated 0D/2D nanocomposite of CsPbBr
3/Bi
2WO
6 via ultrasonic method with intimate contact at the interface to improve the charge separation and transfer. The Bi–Br bonds which is formed at the QDs–NSs interface is responsible for the strong interfacial interaction. The decoration of Bi
2WO
6 with CsPbBr
3 QDs could enhance the CO and CH
4 yield by factor of 9.5 over that of pristine CsPbBr
3 [
64].
The building of 1D semiconductor materials on 2D semiconductors is an efficient strategy for efficient CO
2 conversion. Coupling of TiO
2 nanofibers with light harvesting semiconductors such as MoS
2 nanosheets is an efficient way to overcome the fast recombination of charge carriers and enhance the light absorption efficiency [
65]. The electronic properties of MoS
2 nanosheets can be tuned by control of the thickness. The superior conversion activity of CO
2 into hydrocarbon species, that is, CH
4 and CH
3OH, resulted from the improved light harvesting, sufficient reactive sites for CO
2 adsorption, and the intimate 1D–2D chemical contact between MoS
2 and TiO
2 which could be favorable for facile and efficient charge separation upon photoexcitation [
65]. The increase of the contact area between the two semiconductor nanomaterials is much more favorable to enhance the photocatalytic performance over the photocatalyst. In other words, constructing the 2D/2D interface is favorable for highly separated charge carriers at the interface. Wang et al. prepared 2D/2D heterojunctions by growth of ultrathin tin disulfide (SnS
2) onto TiO
2 nanosheets via a hydrothermal method [
71]. The production yield of CH
4 over SnS
2/TiO
2 was much higher than that of pristine SnS
2 and TiO
2 nanosheets. The reason for such outstanding performance originates from the increment of the contract area between SnS
2 and TiO
2 nanosheets [
71].
The photocatalytic systems with multiple junctions, that is, with multiple interfaces, displays excellent photocatalytic activity toward solar fuels generation compared to one with/without single junction [
67,
144,
145]. Recently, Macyk and coworkers designed two heterointerface-based photocatalyst, TiO
2/C
3N
4/Ti
3C
2, via the interfacial assembly of Ti
3C
2 QDs on the TiO
2/C
3N
4 binary nanocomposite to boost the charge separation and transfer and providing strong redox ability in CO
2 photoreduction reaction [
145]. The as-synthesized composite exhibited enhanced light absorption, suppressed electron-hole recombination, and demonstrated stable photocurrent sensitivity. The fabricated composite could overcome the disadvantage of TiO
2/C
3N
4 nanocomposites with a single junction by providing more efficient transport channels of electrons-hole pairs due to the strong interaction between Ti
3C
2 QDs and TiO
2/C
3N
4 NS. Theoretical studies demonstrated that construction of two interfacial electric fields between TiO
2/C
3N
4 and Ti
3C
2/C
3N
4 is due to electron transfer processes at the two interfaces. The interfacial built-in electric fields can promote the charge carrier separation and the photocatalytic reduction of CO
2 into CO and CH
4. Tonda et al. fabricated another multijunction system with a Bi
2WO
3/RGO/g-C
3N
4 2D/2D/2D architecture using two-step hydrothermal method for utilization in CO
2 and water reduction into useful fuels [
144]. This ternary heterojunction exhibited highly improved characteristics in light harvesting ability, CO
2 adsorption capacity, photocurrent responses, and interfacial contact area. The photoconversion performance of CO
2 over Bi
2WO
3/RGO/g-C
3N
4 was dramatically enhanced toward CH
4 and CO evolution. The performance of Bi
2WO
3/RGO/g-C
3N
4 was 2.5 times higher and 3.8 times higher than those of Bi
2WO
3/RGO and RGO/g-C
3N
4, respectively [
144].
3.2. Modification of Low-Dimensional Nanomaterials
Modification of nanostructured low-dimensional photocatalysts themselves are another beneficial strategy for the enhancement of photocatalytic CO2 conversion because it helps the properties of photocatalysts to be engineered. The modification can be achieved using several approaches such as introduction of surface oxygen vacancies or the formation of a porous structure.
The efficient utilization of the solar spectrum can be controlled by tuning the band structure of the semiconductor using the elemental doping [
19]. Yu et al. synthesized oxygen-doped g-C
3N
4 nanotubes via exfoliation and a 3D g-C
3N
4 curling condensation method [
146]. It was found that the synthesized photocatalyst consisted of curled nanosheets that had a uniform tubular structure with 20–30 nm of diameter. The oxygen atoms can substitute for the C or N atoms in g-C
3N
4 under high temperature oxidation conditions. The oxygen doping of 1D g-C
3N
4 helped the conduction band to be at a more positive potential causing a narrower band gap and efficient light harvesting. This structure exhibited a significant methanol evolution rate of 0.88 µmol∙g
−1∙h
−1 under visible light radiation. Wu et al. synthesized self-doped black TiO
2 nanotubes arrays using a one-step aluminothermic reduction for solar-driven conversion of CO
2 into CO [
147]. It is found that the average diameter of the nanotubes was 75–85 nm with 5–7 nm of wall thickness. The oxygen vacancies can act as active sites for CO
2 molecules for efficient photogenerated charge carrier separation. The visible light absorption of black TiO
2 was largely enhanced by virtue of the oxygen vacancies. The resulted photocatalytic conversion was 185.39 µmol∙g
−1∙h
−1 of CO evolution rate under visible light.
The introduction of defects into semiconductors can improve the photocatalytic activity of CO
2 into solar fuels ascribed to the promotion of photogenerated charge carrier separation and the extended light absorption [
148]. Liu and coworkers prepared Bi
12O
17Cl
2 nanotubes with surface oxygen defects via solvothermal method [
52]. The tubular structure plays crucial role for accelerating the photogenerated charge carrier separation, while the oxygen defects on the surface act as active centers for CO
2 activation. It is found that the absorption of Bi
12O
17Cl
2 nanotubes is improved in the visible region compared to its bulk counterpart. The defective ultrathin tubular structure of Bi
12O
17Cl
2 provides effective CO
2 conversion into CO with production yield of 16.8 times higher than bulk Bi
12O
17Cl
2. The higher photocatalytic conversion rate can be attributed to faster charge separation on the surface of Bi
12O
17Cl
2 nanotubes.
The porosity of nanostructured semiconductors provides an additional feature to increase the surface area of photocatalysts, and subsequently is favorable for the solar-driven reduction of CO
2 into valuable fuels [
149]. Huang et al. used a template-free method to prepare porous g-C
3N
4 with increased surface area [
150]. It is reported that the porous g-C
3N
4 nanotubes had excellent photocatalytic conversion of CO
2 into CO of 40 µmol∙g
−1 within 4 h illumination. The CO yield was higher than that of bulk g-C
3N
4 by a factor of 5.6 originated from the higher surface area of the porous tubular structure, and the improved charge carrier separation and transfer process.