1. Background
Aging is a highly complex process marked by succeeding events that promote modifications in the normal functioning of an individual organism over time [
1,
2]. Numerous factors are involved in the occurrence of aging, comprising, epigenetic modifications, genomic instability, deregulated nutrient-sensing, loss of proteostasis, changed intercellular communication, telomere shortening, and cellular senescence [
3]. Internal aspects include the regular biological activity of the cell, whereas the exterior influences implicate continuing sun-exposure, dietary deficiencies, hormonal difference, and other influences such as pollution and smoking [
4,
5]. Several theories have been hypothesized to define the aging phenomenon [
6]. Denham Harman, in 1950, defined that aging is the effect of a significant synthesis of free radicals [
7]. Free radical is a molecule or an atom with unpaired electrons, that owns the capability to make electronic couples. Free radicals are commonly synthesized during the metabolic reactions under physiological situations [
8], but their production also takes place during contact to ultraviolet (UV) rays, cigarette smoke, and venomous molecules, as well as during emotional stress [
9].
While accurate function of an organism needs metabolic reorganization of numerous chemical building blocks, there is also a damaging outcome that consequence from the accumulated byproducts of those reactions. The highly reactive molecules, created during oxidative metabolism, such as reactive oxygen species (ROS), have the capability to quickly oxidize, and thus injure several molecules. ROS, as well as hydroxyl/peroxyl radicals and peroxides, are formed through the regular processes of metabolism, e.g., oxidative phosphorylation and ATP synthesis. They can also have a helpful role; in fact they can defend the body from opportunistic pathogens, and provoke the production of hormones related to functioning communication between cells [
10]. Disturbed ROS homeostasis, indicated as oxidative stress, is observed with crescent biological age [
11], and it can consequence either from augmented ROS production or reduced capacity to remove ROS [
12,
13].
The oxidative stress contributes to senescence at the cellular level, and oxidative damage to diverse biomolecules takes place over time. ROS have been discovered to significantly contribute to age-related damage at the subcellular level through the destruction of numerous organic molecules including carbohydrates, proteins, DNA, and lipids [
14,
15]. Oxidative stress has been detected during aging [
11], under certain pathological situations [
16,
17,
18,
19], as an effect of contractile action [
20,
21]. Moreover, oxidative stress is often aggravated by a diversity of environmental insults comprising metabolic processing of ingested food, contact with environmental poisons, and infection [
22].
Research carried out in previous years shows that aging is much more malleable than previously thought. Aging is no longer as intractable and mysterious a process, offering new prospects for contributions from other branches of the physiological sciences [
23]. The identification of cellular and molecular hallmarks of aging highlighted the probability for lifestyle-behavioral, comprising nutrition, to improve health span in humans [
24].
In recent decades, the connection between aging and nutrition has been expansively studied in both humans and animals. Numerous food supplements that show antioxidant probability prevent and treat chronic conditions linked to ROS, which results in a healthier and longer life. Scientists have proposed that antioxidants have auspicious properties on both age-related and chronic syndromes, principally cancer and neurodegenerative syndromes [
25].
Natural supplements have antagonistic actions against the body’s inflammatory and degenerative processes and have favorable consequence on the digestive and immune systems, thus ameliorating the quality of life [
5,
26]. Nutraceuticals are natural dietary molecules with medicinal capacities; in fact, the term “Nutraceuticals” is derived from “nutrition” and “pharmaceuticals” [
27]. Agreeing for the foundation for novelty in medicine definition, nutraceuticals are “foods and food products” that have therapeutic importance and offer health positive effects, principally in the prevention and cure of age-related syndromes [
27,
28]. These molecules comprise alimentary supplements, functional foods, and herbal extracts, for example phytochemicals as polyphenols, which provide long-term health benefits [
29,
30].
2. Polyphenols: Anti-Senescence Nutraceuticals
Numerous natural products/nutraceuticals derived from food, plants, and other organisms have been evaluated for their beneficial effects for health.
Polyphenols, organic compounds found copiously in plants, have become an incipient field of interest in nutrition in latest decades. A growing body of research shows that polyphenol consumption may play a vital part in health through the setting of metabolism, weight, chronic syndromes, and cell proliferation, and minor risks of chronic and age-related degenerative syndromes. Animal, human, and epidemiologic searches demonstrate that several polyphenols have antioxidant and anti-inflammatory capabilities that could have preventive and/or therapeutic effects for non-communicable diseases, such as cardiovascular disease, neurodegenerative syndromes, cancer, and obesity [
31,
32,
33].
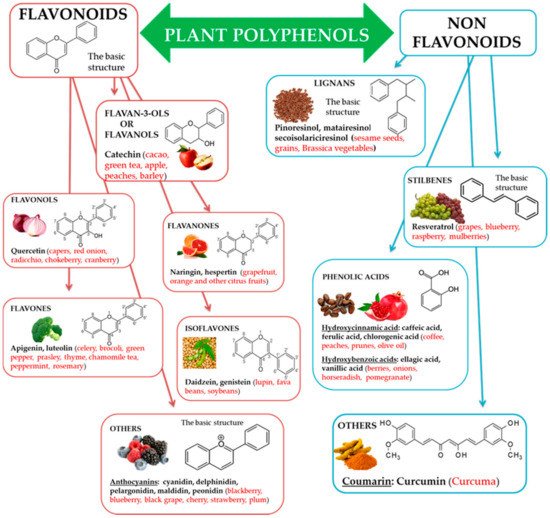
Natural biophenols are a wide group of phytochemicals (over 8000 described so far) found only in the plant kingdom; they are synthesized as secondary metabolites by the plant for defense against the attack by fungi, bacteria, and insects (phytoalexins). They are essential for a diversity of functions in plants, and they are accountable for organoleptic (flavor, color, astringency) and nutritional properties of plant-derived foods [
34,
35]. Chemically phenolic molecules consist of aromatic ring to which one or more OH− substituents are attached [
36]. Despite of their chemical variety, the phenolic complexes are mainly separated into two subgroups: (1) flavonoids and (2) non-flavonoids. The first one is comprised of heterocyclic oxygen which are bonded with two aromatic rings and depends on the quantity of hydrogenation. They can be further divided into six subgroups, i.e., flavanols, flavanones, flavonols, isoflavones, flavones, and other, for example anthocyanins. Meanwhile, the second one contains aromatic rings which are attached to organic acids, like cinnamic and benzoic compounds. Lignans, tannins, stilbenes, and coumarin are also the subgroups of non-flavonoid molecules [
37,
38] ().
Figure 1. Polyphenols, subclasses, basic chemical structures, and representative polyphenolic-food sources (in red).
Polyphenolic compounds have fascinated scientists internationally for their peculiar activities, such as anti-inflammatory, antioxidant power, and anti-carcinogenic properties [
39]. Polyphenolic compounds have been acknowledged for a long time to nutritionists and the numerous scientists, and they are reputed the most potent natural antioxidants [
40]. Since ancient times, humans ingest enormous amounts of polyphenolic compounds within vegetable source. These phytochemicals are present in abundance among fresh fruit and vegetables, especially leafy vegetables with a dark green color and in fruit with shades inclining to red (
Acai berries), in cocoa, tea, and wine. Polyphenol-rich dietary foods are fruits (grapes, apples, berries, pears, and cherries), cereals, tea, red wine, dry beans, coffee, and chocolate, and can behave as active antioxidants [
41,
42]. Several fruits are very abundant in polyphenolic molecules, which are responsible for their taste, aroma, and color [
43]. Apples, blueberries, grapes, raspberries, blackberries, plums, and strawberries are the most abundant in polyphenolic compounds [
31,
44]. Anthocyanins are the most frequently occurring polyphenolic compounds in fruit (especially abundant in colored fruit); then hydroxybenzoic and hydroxycinnamic and acids along with their products, tannins, flavonols, and catechins [
45,
46].
Several natural polyphenols studied for their healthy abilities are curcumin, detected in the tuber of
Curcuma longa Linn (family Zingiberaceae) and an element of the curry; epigallocathechins, markedly epigallocatechin-3-gallate (EGCG), the flavanol discovered in green tea; quercetin and myricetin, flavonols found in tea, onions, cocoa, red wine, and in
Ginkgo biloba. Other polyphenolic compounds have also been examined, with different results; these comprise tannic, ferulic, ellagic, caffeic acid, rutin, kaempferol, apigenin, fisetin, baicalein, luteolin, piceatannol, rottlerin, silibinin, and malvidin [
47].
Most of the natural polyphenols are pigments, typically yellow, red, or purple, and can absorb UV radiation. This ability of natural polyphenols to act as sunscreens can reduce inflammation, oxidative stress, and DNA damaging effects of UV radiation in the skin [
48,
49]. Marine algae-derived polyphenols have been investigated for their photo protective activities. Phlorotannins, as dieckol, phloroglucinol, fucofuroeckol-A, and triphlorethol-A, isolated from marine brown algae, exhibited prominent protective effect against photo damage, induced by UVB radiation [
50,
51]. Thring et al. determined anti-collagenase, anti-elastase, and antioxidant activities of 21 plant extracts and correlated them with the total phenolic content. The white tea extract showed the highest inhibitory activity against enzymes as well as the highest antioxidant activity and phenolic content [
52]. Strawberry extract containing mainly flavonoids and anthocyanins, protected dermal fibroblasts from oxidative stress induced through H
2O
2 [
53]. Studies suggest that polyphenolic extracts can be useful ingredients for both sunscreens and after sun cosmetic products.
In recent decades, special attention has been paid to the anti-proliferative [
54,
55] or anti-oxidative effect of phenolic compounds [
56,
57,
58,
59,
60,
61,
62] with suggestion supporting the probable involvement of polyphenols in the inhibition of various diseases [
63,
64,
65,
66,
67,
68].
Flavonoids are the main antioxidants in the food, and are recognized to preserve against cardiovascular diseases by reducing the oxidation of low-density lipoproteins. Luteolin, apigenin, chrysin, quercetin, datiscetin, morin, myricetin, and kaempferol are some of the most commonly found flavonoids [
69]. Numerous studies, both in vitro and in vivo, have revealed that polyphenols have a brilliant capacity to interfere with our cellular signals, and stimulate a bio-regenerating response. This allows the production of other endogenous antioxidants: oxidative stress advances and this turns into an anti-inflammatory and anti-radical actions [
32].
The properties of polyphenols are also being assessed in terms of communications with the gut microbiota [
70]. Food components are characterized by a two-way communication with microbiota: (i) they can directly control their conformation and (ii) they are catabolized by the intestinal microbes to release metabolites that are more efficient and more easily absorbed than the native molecules [
71]. It is valued that only 5–10% of the total polyphenols intake is absorbed in the small intestine and that 90–95% accumulates in the large intestine, where they undergo enzymatic alteration by the gut microbiota [
72,
73,
74]. Since accumulative evidence supports the hypothesis that the gut microbiota are involved in the progress of human syndromes such as obesity, diabetes, metabolic syndrome, cancer, cardiovascular syndrome, and neurodegenerative diseases, it is conceivable that the defense against age-related syndrome development and progression, hypothesized for some anti-senescence mixtures, is related to the properties of such molecules on the microbiota [
75]. In turn, the gut microbiota can prompt epigenetic variations, as validated in DNA methylation and histone variation of immune system cells.
The gastrointestinal microbiota of healthy human adults involves primarily bacteria belonging to the phyla
Firmicutes and
Bacteroidetes and, to a lesser extent, to
Actinobacteria and
Proteobacteria [
71]. Inflammation may product in a higher level of aerobiosis and making of ROS, which deactivate the strictly anaerobic
Firmicutes and encourage blooms of facultative aerobes, commonly named “pathobionts,” a condition that is habitually observed in the elderly [
76]. The capacity of particular flavonoids, like quercetin, resveratrol, and catechin, to control the gut microbiota has been known in animal models [
77]. Interestingly, apples, which are rich in flavonoids, have been related to a reduction in certain inflammation markers and variations in the gut microbiota of healthy mice [
78].
3. Action of Polyphenols on Some Hallmarks of Aging
Natural polyphenols have been identified as essential plant compounds with anti-aging properties, such as blueberry polyphenols [
79], black tea theaflavins [
80], and procyanidins from apples [
81], resveratrol [
82], curcumin [
83], and epigallocatechin gallate [
84]. Current studies have revealed that polyphenols may modulate a number of phenomena that play a central role in the aging process. These phytochemicals possess a various and interesting pharmacological profile marked by connections with a broad range of biological targets.
Polyphenolic complexes have been revealed to modulate the redox status of cells, to alter cellular signaling, and to help avert the accumulation of injury in long-lived biological molecules such as nucleic acids, lipids, and proteins. This is accomplished both directly, through scavenging of reactive oxygen species, and secondarily, via interaction with transcription factors which coordinate the antioxidant reply. In fact, polyphenols have been revealed to prompt the overexpression of antioxidant enzymes such as superoxide dismutase and catalase [
85]. Polyphenol-rich diets are strong antioxidants that function in vitro and in vivo. Polyphenol compounds such as resveratrol, quercetin, and curcumin have a defensive role against oxidative stress injuries [
86,
87].
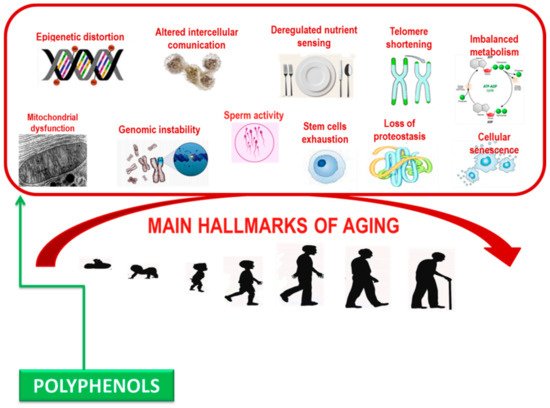
Polyphenols can damp down inflammatory signaling, modulate nutrient sensing pathways, and induce the selective apoptosis of senescent cells. Significantly, these biological processes become dysfunctional with age and are relevant in the pathogenesis of age-related syndrome [
88,
89] (). Deciphering the accurate molecular mechanisms of capability of polyphenols in altering biological phenotypes of age-related syndrome is challenging due to the complexity of biological systems, where multiple diverse biochemical pathways can all contribute to a specific phenotypic outcome such as aging [
90].
Figure 2. Main hallmarks contributing aging.
Over the past numerous decades, research in the biology of aging has attempted to discover “biomarkers” of aging. For example, telomere length has been a highly considered a biomarker of aging, as they shorten as individuals grow older. As proposed by Horvath’s efforts to use methylation markers as a biological clock [
91,
92], high-dimensional protein and/or metabolite profiles might emerge as ideal biomarkers of aging. Several studies suggest polyphenols as modulators of several of these aging indicators.
3.1. Polyphenols and Mitochondria
There is evidence that the accumulation of oxidative mitochondrial DNA damage during normal aging is a risk factor for the development of age-associated neurodegenerative disorders [
93]. It has been revealed that the frequency of point mtDNA mutations augmented about 5-fold during an 80-year lifespan [
94,
95]. The efficiency of mitochondria in producing ATP significantly decreases when humans start aging thereby, allowing the increase of free radicals in these organelles as well as allowing the transit of free radicals through their membranes, in this way damaging other cellular elements [
96]. These changes have facilitated to control the increase in oxidative stress and a reduction in energy production [
11,
97]. Senescence therefore encourages extensive metabolic and bioenergetics modifications [
98]. The removal of dysfunctional mitochondria called mitophagy is critical for cell survival and health, particularly for neurons, as impairments might generally happen with aging [
99,
100].
In fact, two theories of aging regard telomere shortening and exactly mitochondrial DNA (mtDNA) variations and dysfunction. The modern evidence displays the presence of a strong linkage between these two theories suggesting common molecular mechanisms and a complicated telomere-mitochondria interplay during the humans’ aging [
101].
As for the mitochondria, several information prove that polyphenols as resveratrol, curcumin, oleuropein, and hydroxytyrosol exert their positive abilities via the improved incentive of mitophagy intermediaries. The molecules stimulate the up-regulation of mitophagy and so increase the degradation of damaged mitochondria as well as the synthesis of new ones. Resveratrol encouraged peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and mitochondrial transcription factor A (mtTFA) expression to augment mitochondrial biogenesis and stimulus proteins expression to control the balance of mitochondrial fission/fusion, thus preserving mitochondrial homeostasis [
102,
103].
Furthermore, all of the mentioned bioactive food molecules also own anti-oxidative abilities, which have a very important biological role in that since oxidative stress is deemed as one of the principal mediators of mitochondrial damage and decay in mitophagy occurring during aging [
104]. The efficient elimination of not functional organelles and aggregated proteins is therefore basic to avert raised cellular stress and degeneration. The defensive action of antioxidants, ROS scavengers, and stimulators of mitophagy appears to be the focal mechanism of longevity and of reducing the risk for degenerative syndromes [
99,
101].
Additional of the most critical elements in aging is the accumulation of genetic damages throughout life. DNA solidity is compromised invariably by exogenous factors such as physical, biological, and chemical injuries. Further, DNA integrity is at risk by endogenous factors such as unrepaired defects in replication (point mutations, translocations, chromosomal gains, and losses), and telomere shortening. Oxidative DNA damage seems to be serious for aging, age-related syndromes and cancer. ROS and products of lipid peroxidation can have an effect on both genomic and mitochondrial DNA, leading to several types of DNA damage: double- and single-strand breaks, intra- and interstrand DNA crosslinks, DNA-adduct formation, DNA base and deoxyribose changes [
105]. If the DNA mutations caused by free radicals are not corrected by particular repair mechanisms, these alterations remain even after numerous consecutive replication, transcription, and translation cycles [
106].
3.2. Polyphenols and Telomeres
Shortening of telomeres is a well-known theory linked to aging. As a consequence of the problem of final replication, telomeres shrink in each generation of the cell until they reach a considerable length in the crisis phase of aging [
107]. At this phase, cell division reduces speed noticeably, determining slow cell death. This phenomenon is called “replicative mortality.” The cells involved in growth, development, and reproduction (stem cells, eggs, and spermatozoa) synthesize large quantities of the enzyme telomerase, an enzyme involved in preserving the length of telomeric DNA [
108]. Instead, most adult cells express little or none of this enzyme, producing these cells to age and ultimately die [
101].
Levels of oxidative stress, antioxidants, mitochondrial alteration, inflammation, shortening of telomeres, and gene mutations all have a critical role in defining cellular aging. Studies propose that oxidative stress and the free radicals formed by it play an indispensable role in telomere shortening through reducing the action of telomerase or telomeric repeat-binding factor 2 (TRF-2) levels [
87]. Shortening the telomere length harms health and leads to genomic variability and consequently jeopardizes the function of the cell cycle, and the cells enter the aging and apoptotic phases [
87,
109].
Nowadays, investigators are trying to increase telomerase activity and stabilize telomere length and prolong life by using antioxidant supplements such as polyphenols [
110]. Studies and indication propose that polyphenols, with their antioxidant and anti-inflammatory capabilities, can affect telomere length and prevent shortening as far as possible. The antioxidant effects of diet on telomere function show that diet is a significant factor in determining telomere length status. A research indicated that leukocyte telomere length is considerably improved in subjects who take Mediterranean diet (MedDiet), rich in olive oil [
111]. Proanthocyanidins and procyanidins are polyphenols found in grape seed extract. These polyphenols are powerful free radical scavengers, own anti-inflammatory capabilities decrease apoptosis, and impede hydrogen peroxide induced chromosomal injury in human lymphoblastic cells. Their free radical scavenging ability is 20 times more actual than vitamin E and 50 times more effective than vitamin C [
112]. EGCG, and quercetin with strong antioxidant effect, may impede cardiac myocyte apoptosis by averting telomere shortening and TRF-2 loss [
113]. Tea is rich in polyphenols, and other phytochemicals; a cross-sectional study of Chinese men and women found that elderly Chinese men had a helpful association with telomere length [
114].
Therefore, studies propose that polyphenols, with their antioxidant and anti-inflammatory abilities, can affect telomere length and prevent shortening as far as probable and thus have powerful anti-aging capabilities [
87].
Genetics is clearly significant in determining cellular aging in vitro and in vivo, and part of organismal aging may be dependent on cell division, with total cellular lifespan measured by the number of cell divisions (i.e., generations), not essentially by chronological time. This means that there is an intrinsic process occurring during cell growth which culminates in the interruption of cell division. If cellular age is controlled by a genetically determined counting programmer that controls the number of cell divisions, then it is central to define and understand the molecular pathways and regulation of this mechanism [
109]. The studies have recognized the genes that can extend life expectancy and decrease age-related syndromes, including the Klotho gene. Polyphenols can influence intracellular function through activation of the Klotho gene, which induces the transcription factors, insulin-like growth factor 1 (IGF-1), and transforming growth factor (TGF-1β) [
115].
3.3. Polyphenols and Sirt-1
Several reports highlighted that dietary supplementation of polyphenols may defend against neurodegenerative, cardiovascular, metabolic syndromes, inflammatory, and cancer by enhancing Sirt-1 deacetylase action. Sirtuins are a class of nutrient-sensitive epigenetic information regulators, including in promoting mammalian health, modulating cellular senescence and lifespan, and Sirt1 is called the longevity gene. Sirt1 is a (NAD
+)-dependent deacetylase that targets a number of transcription factors, such as fork head box transcription factor (FOXO) 1, 3, and 4, p53, nuclear factor NF-κB, and peroxisome proliferator-activated receptor gamma co-activator 1 (PGC-1), moderating in turn a number of cellular stress adaptive responses. Sirt1 can deacetylase p53 in a NAD+-dependent manner to impede p53 transcription, modulating pathways involved in cellular and organismal aging [
116]. Significantly, sirtuins are themselves controlled by diet and environmental stress [
117,
118,
119]. Sirtuins impact multiple cellular pathways responsible for regulating gene expression, metabolism, DNA repair, apoptosis, and aging. Activating this pathway can increase life expectancy.
Resveratrol, curcumin, quercetin, tannins, and catechins may also be cited as molecules that increment sirtuins’ actions [
120,
121,
122]. Resveratrol has been found to ameliorate brain health through numerous signaling pathways mechanisms through Sirt-1. The regulatory mechanisms include anti-inflammatory, anti-oxidative, anti-apoptotic processes and autophagy regulation, as well as increases in cerebral blood flow and enhancements in the plasticity of synaptic pathways [
123]. Administration of polyphenols quercetin, silymarin, and naringeninin determined restorative actions on cognition and motor coordination in rats. These polyphenols inverted the age-induced insufficiencies in mono-aminergic neurotransmitters and amplified Sirt-1 levels and reduced NF-κB levels in hippocampus [
124].
Chronic treatments with catechins, polyphenols present in many dietary foods, as gooseberries, apples, grape seeds, blueberries, strawberries, kiwi, red wine, green tea, cocoa, beer, cacao liquor, and chocolate, increase hippocampal Sirt-1 levels improving cognition in aged rats [
125]. Therefore, polyphenols may defend Sirt-1 due to their antioxidant abilities and, in turn, moderate proteins affected by Sirt-1 action [
122].
3.4. Polyphenols and Male Fertility
Aging has an important impact on male fertility, but the mechanisms diminishing fertility rate in elderly subjects are still poorly understood. The well-known endocrine route that sustains the progression of spermatogenesis and spermatozoa differentiation [
126,
127] declines due to steroid genesis defects. In addition, during the aging process morphological and functional alternations affect the testis, semen quality declines with changes in sperm morphology and concentration, and this causes defects in the acquisition of sperm motility [
128]. At molecular level, sperm DNA damage, alteration in chromatin architecture mainly due to defective protamination, occurs. In parallel, the deregulation of epigenetic marks (i.e., non-coding RNA profile) in both spermatozoa and seminal plasma may affect the subsequent embryonic development and offspring health [
129,
130]. Oxidative stress, together with the decrease in antioxidant activity and mitochondria dysfunctions, is the main cause of testicular and sperm damage being ROS notably responsible for spermatogenesis failure, apoptotic loss of both germ and somatic cells, oxidative DNA damage, failure in gene expression and post-transcriptional gene regulation, APT depletion leading to insufficient axonemal phosphorylation in sperm tail, lipid peroxidation, and loss of sperm motility and viability, among the others [
131,
132].
Therefore, controlled antioxidant supplementation may be useful to preserve sperm quality along the life-span [
132,
133]. Furthermore, in animal models and humans antioxidants resulted to be useful to preserve semen quality before cryopreservation and after thawing [
133] for in vitro fertilization (IVF) [
134]. In this respect, several studies reported the effects of polyphenols supplementation on sperm quality parameters. For example, tea polyphenols, known as catechins, decreased the apoptosis rate of spermatogenic cells in rats with experimental varicocele [
135]. Consistently, the ad libitum administration of 2% and 5% aqueous extract of green tea for 52 days increased sperm concentration and viability in rats, [
136]. In the same study, the authors revealed spontaneous acrosome reaction and morphological changes in testis and epidydimis, with increased cauda epididymis epithelial height and decreased diameter and epithelial height of seminiferous tubules [
136]. Similar effects on sperm vitality, motility, acrosome reaction, and morphological parameters in the seminiferous tubules and epididymis were observed following black tea administration [
137]. Both green tea and black tea decreased serum levels of alanine transaminase and aspartate transaminase but black tea only increased creatinine levels [
137]
Aqueous leaf extract of
Moringa oleifera, the “miracle tree” containing a great number of bioactive compounds including polyphenols [
138], reduced intracellular ROS production, DNA fragmentation and acrosome reaction without any effect on sperm motility, vitality, mitochondrial membrane potential and capacitation in human spermatozoa in vitro [
139]. Quercetin improves the quality of cryopreserved human, dog and bull semen [
140,
141,
142] and exerts protective effects against heavy metals induced oxidative injury in goat sperm and zygotes [
143]. In vitro, resveratrol has protective effect on the sperm functions affecting motility, plasma zinc concentration, and acrosin activity in male infertility induced by body weight excess and obesity [
144]. It also counteracts the detrimental effects of the polycyclic aromatic hydrocarbon benzo-α-pyrene on human sperm [
145], and significantly improves the fertilization capacity in humans and animal models [
146,
147,
148]. A comparative analysis of resveratrol and EGCG has been carried out on thawed boar spermatozoa revealing an increase in the total efficiency of fertilization, for both molecules [
146]. Then, polyphenols may be useful to preserve spermatozoa quality parameter with potential application in IVF.
3.5. Polyphenols and Inflammation, Apoptosis, and Autophagy
Other essential changes in the aging process comprise chronic inflammation of the body’s cytokines (such as interleukin (IL)-6, tumor necrosis factor (TNF)-α). Anti-inflammatory action of polyphenols such as catechin, apigenin, luteoloside, ellagic acid, and rutin, has been detected in acute and chronic inflammation. The molecular mechanisms of polyphenols are associated with inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathways [
149]. Resveratrol augmented insulin sensitivity, AMP-activated protein kinase (AMPK), peroxisome proliferator-activated receptor-c coactivator 1α (PPAR-co-1α) activity and anti-inflammatory microRNAs [
150,
151]. Polyphenolic compounds perform the anti-obesity action by inhibiting or decreasing lipid synthesis in adipocytes, moderating lipogenesis, decreasing inflammation and oxidative stress, and stimulating AMPK through stopping ATP synthesis [
152].
Triggering programmed cell death is an effective mechanism to prevent cellular aging. In the process of apoptosis, older cells are destroyed and replaced with young cells. However, this protection against aging has many risks. During aging, inadequate cell death origins cancer to spread, and extreme cell death leads to tissue atrophy, connected with reduced life-span [
153,
154]. Polyphenols can constrain muscle atrophy and damage to the immune system from inhibiting the apoptosis process while enhancing this process is effective in clearing cancer cells. Nevertheless, there is a great deal of disagreement regarding the capability of these phytochemicals to promote or reverse apoptosis, and the relationship between apoptosis and the aging process needs further revisions and clarification.
Autophagy is an internal process that aids in lysosomal lack and removal of old and unwanted cell molecules, including proteins, lipid droplets, ribosomes, and other organelles, protecting cell homeostasis and survival under metabolic stress. Hence, autophagy protects the general health of the host, particularly in pathological situations such as cancer and diabetic cardiomyopathy. Numerous bioactive polyphenols, such as isoflavones and curcumin [
155], are capable of causing autophagy. Optimal concentrations of EGCG were able to cause autophagy, anti-inflammatory effect [
156], destroy lipid droplets in endothelial cells, and stimulate the degradation of endotoxins with anti-inflammatory effects [
156]. Numerous studies suggest that the activation of autophagy by numerous polyphenols should contribute to their neuroprotective action. Tea polyphenols can activate autophagy by various mechanisms, such as the mammalian target of the rapamycin pathway (mTOR) [
157]. Treatment with EGCG can cause autophagy, as it reduces the action of negative autophagy regulators that control apoptosis. In other words, EGCG is capable of amplifying autophagy, thereby slowing cell death mediated by apoptosis and thus increasing cell viability [
158].
3.6. Polyphenols, Nrf2, and Proteostasis
One of the elegant mechanisms that cells have adapted to protect themselves against oxidative stress and other insults is Nrf2 pathway and the binding of this master transcriptional regulator with antioxidant response element (ARE) in the regulatory region of many genes, which leads to the expression of several enzymes with antioxidant and detoxification capacities. The nuclear factor E2-related factor 2 (Nrf2) and its invertebrate homologs have emerged as master regulators of cellular detoxification responses and redox status. These stress-sensing transcription factors function both in situations of acute challenge and as regulators of baseline antioxidant activity. The oxidative stress theory of aging posits that oxidative damage to biological macromolecules is a key driver of aging, and that, conversely, mechanisms that delay the accumulation of oxidation products in the cells and tissues of an organism can promote longevity. Consistent with this tenet and the established role of Nrf2 and its invertebrate homologs as master regulators of antioxidant gene expression, a number of studies support a function for the Nrf2 pathway in the regulation of lifespan. In both
C. elegans and
D. melanogaster, the genetic activation of the Nrf2 signaling can cause significant increases in longevity [
159]. Under normoxic conditions, Nrf2 levels are low, predominantly due to binding to the negative regulator KEAP1 (Kelch-like ECH-associated protein 1), which facilitates Nrf2 ubiquitination and proteasomal degradation [
160]. During increased oxidative stress, oxidative cysteine modification of KEAP1 alters its conformation, resulting in diminished binding to Nrf2. Nrf2, no longer subject to degradation, translocates to the nucleus where it binds to the ARE upstream of cytoprotective genes, e.g., NAD(P)H quinone oxidoreductase 1, glutathione S-transferase, and glutathione reductase [
161], inducing their expression. The Keap1-Nrf2/ARE signaling pathway is an important defense system against exogenous and endogenous oxidative stress injury. These factors act to lower ROS and oxidative stress, while simultaneously reducing the cysteines in Keap1 and subsequently re-establishing baseline equilibrium of Nrf2 activity. Several studies have shown that polyphenols can induce Nrf2 function in different models [
162,
163]. For example, in endothelial cells resveratrol has anti-inflammatory effects that appear to be mediated by the induction of Nrf2 [
159]; in mice, resveratrol improved renal function by activation of the Nrf2 and Sirt1 signaling pathways, ameliorating oxidative stress and mitochondrial dysfunction [
162,
164].
As well coordinating the antioxidant response, activation of Nrf2 has been demonstrated to increase proteasomal activity, allowing cells to control protein levels by regulated degradation. Activation of Nrf2 increases the expression and activity of the proteasome in a Nrf2-dependant manner [
90,
165].
Another molecular mechanism that prompts senescence is impaired protein homeostasis or proteostasis [
166,
167]. This is thought to be due at least in part to an increase in the accumulation of errors in translation, splicing, or molecular misreading, and to an increased production of oxidized and carbonylated proteins, and therefore systems are necessary which regulate and preserve a functional cellular protein pool. Proteostasis is a network of quality-control processes including protein clearance mechanisms that constrain the toxicity of misfolded proteins. Ensuring cellular protein homeostasis requires specific control of protein synthesis, folding, conformational maintenance, and degradation. A complex and adaptive proteostasis network coordinates these processes with molecular chaperones of diverse classes and their regulators functioning as major players. The most important systems for these removal processes are the “ubiquitin-proteasomal system” (UPS), the central proteolytic machinery of mammalian cells, mainly responsible for proteostasis, as well as the “autophagy-lysosomal system,” which mediates the turnover of organelles and large aggregates. Many age-related pathologies and the aging process itself are accompanied by a dysregulation of UPS, autophagy, and the cross-talk between both systems [
166,
167]. Failure to destroy the unfold proteins by proteostasis system will lead to the amassing and aggregation of these proteins and ultimately induce aging [
168,
169]. Although the protein quality-control networks ensure proteostasis under basal conditions, on conformational stress, such as increases in temperature or exposure to oxidative agents, many additional proteins become prone to misfolding, with proteins comprising the metastable sub-proteome being particularly vulnerable [
166,
170]
Irreversibly oxidized proteins must be degraded and replaced by de novo synthesized ones in order to maintain functionality and proteostasis of a cell. In the case a protein is oxidatively modified/damaged by ROS in an ongoing process, it undergoes a transition from slight functional decrease and increased solubility to a completely dysfunctional, unfolded, and insoluble structure that may be even resistant to mammalian proteases due to covalent cross-linking, depending on the amount of oxidative modification [
167]. In order to counteract oxidative damage of cellular structures in redox-shifts, inflammation or oxidative stress, exceeding the “basic” amount of ROS produced in normal cellular function, there are powerful systems that can be induced, increasing the antioxidant capacity of the cell. If most important cellular ROS-scavenging enzymes are not sufficient to prevent a cellular redox shift, molecular redox-sensors such as Keap1/Nrf2 can be activated very quickly [
167].
Polyphenolic compounds may enhance the efficacy of associated protein degradation by the proteasome and autophagy, and weaken oxidative stress [
47,
171]. Although these molecules act on numerous biochemical pathways, their activity in regulating the protein degradation mechanisms at different stages may be a suggestive therapy to stop the increase of misfolded proteins [
172,
173].
The ever-growing interest and public awareness surrounding the potential benefits of natural health products and polyphenols, in addition to their widespread availability and accessibility through nutritional supplements and fortified foods, has led to increased consumption. Foods can be fortified with polyphenols; dietary supplements that contain high doses of polyphenols can be developed. Some studies purport that excessive polyphenol consumption may have negative health effects in some sub-populations [
174,
175,
176]. In such cases, toxicological testing may be required to ensure safe levels of intake. Then, too much may not be good and thus, dietary polyphenols may be beneficial in the correct amounts. The risk of consuming high doses of polyphenols from naturally polyphenol-rich foods is low. Therefore, the advice is to one should be content with eating a good diet for now and to take polyphenolic-food.