Bone metastasis remains a major cause of death in cancer patients, and current therapies for bone metastatic disease are mainly palliative. Bone metastases arise after cancer cells have colonized the bone and co-opted the normal bone remodeling process.
- bone metastases
- radiopharmaceuticals
1. Introduction
Since bone metastasis is one of the major causes of death of cancer patients, eradicating cancer-induced bone diseases represents one of the greatest challenges of modern health care. Thus, there is a critical need to integrate our current understanding of cancer metastasis with emerging concepts in bone biology to advance our understanding of cancer-induced bone diseases, with the goal of improving treatment strategies and clinical outcomes, while reducing the financial difficulties experienced by patients. The treatment strategies for bone metastases are somewhat unique when compared to those for other metastases. Normally, the treatment strategies for both primary and metastatic tumors are similar—targeting the tumors themselves or inducing the immune system surrounding the tumors. However, for bone metastases, the treatments target the function of the metastatic organ, which is bone (an organ that continuously remodels throughout life by coupling osteoclast and osteoblast activity, which is called bone remodeling [1]). It has been suggested that the cells involved in bone remodeling (e.g., osteoclasts, osteoblasts, and osteocytes) and bone metastatic cancer cells interact with each other, and this crosstalk between bone-related cells and bone metastatic cancer cells stimulates further bone metastatic progression, known as “the vicious cycle of bone metastases” [2]. It is therefore natural to target bone remodeling to interfere with this cycle. Indeed, bisphosphonate and denosumab, a human monoclonal anti-receptor activator of nuclear factor κB ligand (RANKL) antibody, which decreases osteoclastic activity, have been used as treatments for bone metastases [3][4]. These treatments have been effective in reducing the painful complications of bone metastases but ultimately fail to improve the overall survival of cancer patients with bone metastases [3][4]. However, recent clinical trials indicate that an alpha-particle-emitting radiopharmaceutical radium-223 dichloride (223RaCl2), which targets hydroxyapatite or osteoblastic bone metastatic lesions, improves the overall survival of prostate cancer patients with bone metastases. Importantly, to date, this is the only bone-targeted treatment modality that can prolong the survival time of cancer patients with bone metastases, although several combinations of systemic treatments (e.g., hormone therapies and chemotherapies) are known to enhance the overall survival of metastatic castration-resistant prostate cancer patients, including patients with bone metastases [5]. Although 223RaCl2’s success holds promise for alpha-particle-emitting radiopharmaceuticals for bone metastatic disease and has renewed interest in the development of these therapies [6][7][8][9][10][11][12][13][14][15][16][17][18], little is known as to the targeted treatment strategies for bone metastatic disease using alpha-particle-emitting radiopharmaceuticals.
2. The Biology of the Vicious Cycle of Bone Metastases
Bone is continuously renewed throughout life to maintain its structural integrity. Bone is composed of three parts: compact bone, trabecular bone, and bone marrow. Compact bone is a hard, solid bone tissue and forms the outside layer of bone. Trabecular bone (or spongy bone) and bone marrow are found in the inside of bones. A part of trabecular bone eventually converts into compact bone. The bone marrow is composed of two distinct stem cell lineages, cells of hematopoietic origin and those of mesenchymal origin. Hematopoietic stem cells (HSCs) give rise to all blood cell types, including macrophages that differentiate into osteoclasts, while mesenchymal stem cells (MSCs) are responsible for the generation of stromal cells, osteoblasts, and osteocytes [19]. Additionally, these cells of mesenchymal origin are crucial for trabecular bone development. Interestingly, these two cell lineages interact with each other to maintain each other’s functions.
Once these tumor cells have effectively seeded on the bone marrow, they begin to proliferate and interact with the cells involved in bone remodeling (e.g., osteoclasts, osteoblasts, and osteocytes) through paracrine and juxtacrine signaling events. This interaction creates an imbalance in the normal bone remodeling process in what has been termed the vicious cycle of bone metastases (Figure 1) [20].
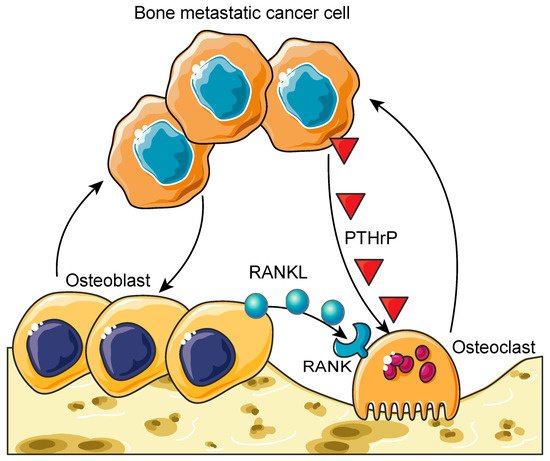
Figure 1. The vicious cycle of bone metastases. Bone metastatic cancer cells hijack the healthy bone remodeling process to create a suitable microenvironment for them to grow. Cancer cells induce hyper-osteoclastogenesis by activating osteoclasts through the secretion of parathyroid-hormone-related peptide (PTHrP). This process leads to osteolytic bone lesions and provides bone metastatic cancer cells more space to grow. On the other hand, cancer cells can over-activate osteoblasts, resulting in osteoblastic bone lesions. These hyper-activated osteoblasts also stimulate osteoclastogenesis through the receptor activator of the nuclear factor κB ligand (RANKL) (secreted from osteoblasts)/RANK (expressed on osteoclasts) axis. Furthermore, these hyper-activated osteoblasts and osteoclasts enhance the growth and survival of bone metastatic cancer cells. This process is called the vicious cycle of bone metastasis. Graphics adapted from Smart Servier Medical Art (https://smart.servier.com/, accessed on 18 March 2021).
3. Targeted Radiopharmaceuticals for Bone Metastases
To date, the strategy for integrating systemic radiotherapies into the treatment plans of cancer patients with bone metastases has relied on bone metabolism to target bone metastatic disease. While successful, the results of this strategy have been primarily palliative, since the bone metastatic cancer cells are not specifically targeted by the radiopharmaceutical. As a result, efforts are being made to expand this strategy by developing agents that can selectively target biomarkers that are over-expressed on the cancer cells within the bone metastatic microenvironment, with the goal of improving therapeutic efficacy and patient outcomes. PSMA is known to be highly expressed on prostate cancer cells at the primary tumor and within visceral and bone metastases [21]. PSMA-617, a small molecule that binds to PSMA with high affinity, was designed to target PSMA-expressing prostate cancer cells [22]. In a recent single-arm, single-center, phase II trial, 177Lu-PSMA-617 was administered to men with metastatic castration-resistant prostate cancer (n =30) [23]. After four cycles of radiotherapy, 57% of these patients experienced minimal toxicity and achieved a greater-than-50% reduction in prostate-specific antigen (PSA) levels, which is one of the clinical surrogate markers for prostate cancer treatment response [23]. Based on these promising results, an international prospective open-label randomized phase III trial comparing the treatment efficacy between 177Lu-PSMA-617 and the best standard of care in men with metastatic castration-resistant prostate cancer (VISION trial, NCT03511664) is currently underway [24], and as of 23 March 2021, the initial result that 177Lu-PSMA-617 significantly improves the overall survival and radiographic progression-free survival of PSMA-positive metastatic castration-resistant prostate cancer patients was announced (https://www.novartis.com/news/media-releases/novartis-announces-positive-result-phase-iii-study-radioligand-therapy-177lu-psma-617-patients-advanced-prostate-cancer, (accessed on 1 April 2021)).
Despite the promise of 177Lu-PSMA-617 therapy, approximately 30% of patients do not respond to this treatment [25]. These observations have led to the investigation of the use of 225Ac-PSMA-617 as an alternative alpha-particle-emitting radiotherapy for refractory patients [25][26][27]. Actinium-225 (225Ac: t1/2 = 10 d; Eαmax = 6–8 MeV) is a radioactive metal of the actinide series. Similar to 223RaCl2, 225Ac has a relatively long half-life and emits four α++- and two β−-particles per nuclear decay. However, unlike 223RaCl2, 225Ac can be linked to a variety of targeting ligands [28][29][30][31][32][33][34][35]. In the early stage of the development of 225Ac-PSMA-617, two patients received this therapy [25]. The first patient exhausted conventional chemotherapies, bone remodeling therapy, ADTs, and six cycles of 223RaCl2 therapy. The patient received three cycles of approximately 10 MBq (100 kBq/kg body weight) of 225Ac-PSMA-617. After 8 weeks, all visible visceral and bone metastases had decreased to a size that was below the limit of detection of clinical imaging scanners, while PSA levels had decreased from 3000 to 0.26 ng/mL. Similar to the first patient, the second patient was refractory to conventional chemotherapies and ADTs and failed to respond to several cycles of 177Lu-PSMA-617 therapy. This patient received three cycles of 225Ac-PSMA-617 therapy (100 kBq/kg body weight) at bimonthly intervals. After completing the three cycles, the patient experienced complete remission, as indicated by the results of the restaging PSMA-PET/CT scans (Figure 2). Similar to the first patient, no relevant hematological toxicities were observed, although both subjects did experience moderate xerostomia.
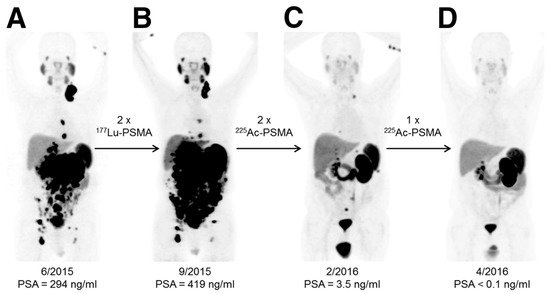
Figure 2. An example of the effective response of a prostate cancer patient to 225Ac-PSMA-617 treatment. This research was originally published in the Journal of Nuclear Medicine [25]. Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, Kopka K, Apostolidis C, Haberkorn U, and Morgenstern A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J Nucl Med. 2016;57(12):1941-1944. © SNMMI. 68Ga-PSMA-11 PET/CT scans of patient B in this manuscript (a prostate cancer patient who presented with peritoneal carcinomatosis and liver metastases that were progressive under 177Lu-PSMA-617 therapy). In comparison to the initial tumor spread (A), restaging after 2 cycles of β-emitting 177Lu-PSMA-617 presented progression (B). In contrast, restaging after second (C) and third (D) cycles of α-emitting 225Ac-PSMA-617 presented an impressive response. Adapted from ref. [25].
This entry is adapted from the peer-reviewed paper 10.3390/molecules26082162
References
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396.
- Guise, T.A. The vicious cycle of bone metastases. J. Musculoskelet. Neuronal. Interact. 2002, 2, 570–572.
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139.
- Fizazi, K.; Carducci, M.; Smith, M.; Damiao, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822.
- McCain, J. Drugs that offer a survival advantage for men with bone metastases resulting from castration-resistant prostate cancer: New and emerging treatment options. Pharm. Ther. 2014, 39, 130–143.
- Nguyen, N.C.; Shah, M.; Appleman, L.J.; Parikh, R.; Mountz, J.M. Radium-223 Therapy for Patients with Metastatic Castrate-Resistant Prostate Cancer: An Update on Literature with Case Presentation. Int. J. Mol. Imaging 2016, 2016, 2568031.
- Vogelzang, N.J.; Coleman, R.E.; Michalski, J.M.; Nilsson, S.; O’Sullivan, J.M.; Parker, C.; Widmark, A.; Thuresson, M.; Xu, L.; Germino, J.; et al. Hematologic Safety of Radium-223 Dichloride: Baseline Prognostic Factors Associated with Myelosuppression in the ALSYMPCA Trial. Clin. Genitourin Cancer 2017, 15, 42–52.
- Wadas, T.J.; Pandya, D.N.; Solingapuram Sai, K.K.; Mintz, A. Molecular targeted alpha-particle therapy for oncologic applications. AJR Am. J. Roentgenol. 2014, 203, 253–260.
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in targeted alpha-particle therapeutic applications. Curr. RadioPharm. 2011, 4, 306–320.
- Yamazaki, H.; Nakamura, S.; Suzuki, G.; Yoshida, K.; Yoshioka, Y.; Koizumi, M.; Ogawa, K. Hypofractionated Radiotherapy for Localized Prostate Cancer: A Challenging Accelerated Hypofractionated Radiotherapy. Anticancer Res. 2015, 35, 5167–5177.
- Lewis, B.; Chalhoub, E.; Chalouhy, C.; Sartor, O. Radium-223 in Bone-Metastatic Prostate Cancer: Current Data and Future Prospects. Oncology (Williston Park) 2015, 29, 483–488.
- de Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy-How to Deal with Recoiling Daughters? Pharmaceuticals (Basel) 2015, 8, 321–336.
- Guerard, F.; Barbet, J.; Chatal, J.F.; Kraeber-Bodere, F.; Cherel, M.; Haddad, F. Which radionuclide, carrier molecule and clinical indication for alpha-immunotherapy? Q. J. Nucl. Med. Mol. Imaging 2015, 59, 161–167.
- McGann, S.; Horton, E.R. Radium-223 dichloride: A novel treatment option for castration-resistant prostate cancer patients with symptomatic bone metastases. Ann. Pharmacother. 2015, 49, 469–476.
- Qi, W.X.; Fu, S.; Zhang, Q.; Guo, X.M. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: A systematic review and meta-analysis. Radiother. Oncol. 2015, 114, 289–295.
- Buroni, F.E.; Persico, M.G.; Pasi, F.; Lodola, L.; Nano, R.; Aprile, C. Radium-223: Insight and Perspectives in Bone-metastatic Castration-resistant Prostate Cancer. Anticancer Res. 2016, 36, 5719–5730.
- Cordier, D.; Krolicki, L.; Morgenstern, A.; Merlo, A. Targeted Radiolabeled Compounds in Glioma Therapy. Semin. Nucl. Med. 2016, 46, 243–249.
- Ettari, R.; Previti, S.; Bitto, A.; Grasso, S.; Zappala, M. Immunoproteasome-Selective Inhibitors: A Promising Strategy to Treat Hematologic Malignancies, Autoimmune and Inflammatory Diseases. Curr. Med. Chem. 2016, 23, 1217–1238.
- Laurenti, E.; Gottgens, B. From haematopoietic stem cells to complex differentiation landscapes. Nature 2018, 553, 418–426.
- Cook, L.M.; Shay, G.; Araujo, A.; Lynch, C.C. Integrating new discoveries into the “vicious cycle” paradigm of prostate to bone metastases. Cancer Metastasis Rev. 2014, 33, 511–525.
- Hupe, M.C.; Philippi, C.; Roth, D.; Kumpers, C.; Ribbat-Idel, J.; Becker, F.; Joerg, V.; Duensing, S.; Lubczyk, V.H.; Kirfel, J.; et al. Expression of Prostate-Specific Membrane Antigen (PSMA) on Biopsies Is an Independent Risk Stratifier of Prostate Cancer Patients at Time of Initial Diagnosis. Front. Oncol. 2018, 8, 623.
- Sun, M.; Niaz, M.O.; Nelson, A.; Skafida, M.; Niaz, M.J. Review of 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer. Cureus 2020, 12, e8921.
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833.
- Rahbar, K.; Bodei, L.; Morris, M.J. Is the Vision of Radioligand Therapy for Prostate Cancer Becoming a Reality? An Overview of the Phase III VISION Trial and Its Importance for the Future of Theranostics. J. Nucl. Med. 2019, 60, 1504–1506.
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944.
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Tripathi, M.; Seth, A.; Bal, C. Efficacy and safety of (225)Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics 2020, 10, 9364–9377.
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. (225)Ac-PSMA-617 for Therapy of Prostate Cancer. Semin. Nucl. Med. 2020, 50, 133–140.
- Deblonde, G.J.; Abergel, R.J. Active actinium. Nat. Chem. 2016, 8, 1084.
- Vasiliev, A.; Severin, A.; Lapshina, E.; Chernykh, E.; Ermolaev, S.; Kalmykov, S. Hydroxylapatite particles as carriers for 223Ra. J. Radioanal. Nuclear Chem. 2016, 311, 1503–1509.
- McDevitt, M.R.; Ma, D.; Simon, J.; Frank, R.K.; Scheinberg, D.A. Design and synthesis of 225Ac radioimmunopharmaceuticals. Appl. Radiat. Isot. 2002, 57, 841–847.
- Sofou, S.; Kappel, B.J.; Jaggi, J.S.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Enhanced retention of the alpha-particle-emitting daughters of Actinium-225 by liposome carriers. Bioconjug. Chem. 2007, 18, 2061–2067.
- Antczak, C.; Jaggi, J.S.; LeFave, C.V.; Curcio, M.J.; McDevitt, M.R.; Scheinberg, D.A. Influence of the linker on the biodistribution and catabolism of actinium-225 self-immolative tumor-targeted isotope generators. Bioconjug. Chem. 2006, 17, 1551–1560.
- Borchardt, P.E.; Yuan, R.R.; Miederer, M.; McDevitt, M.R.; Scheinberg, D.A. Targeted actinium-225 in vivo generators for therapy of ovarian cancer. Cancer Res. 2003, 63, 5084–5090.
- Davis, I.A.; Glowienka, K.A.; Boll, R.A.; Deal, K.A.; Brechbiel, M.W.; Stabin, M.; Bochsler, P.N.; Mirzadeh, S.; Kennel, S.J. Comparison of 225actinium chelates: Tissue distribution and radiotoxicity. Nucl. Med. Biol. 1999, 26, 581–589.
- Deal, K.A.; Davis, I.A.; Mirzadeh, S.; Kennel, S.J.; Brechbiel, M.W. Improved in vivo stability of actinium-225 macrocyclic complexes. J. Med. Chem. 1999, 42, 2988–2992.
