Site-directed spin labeling (SDSL) is a molecular biology technique in which paramagnetic spin labels are incorporated into the specific site of bio-macromolecules for investigation of structure and dynamic properties using electron paramagnetic resonance (EPR) spectroscopy. In SDSL, all native nondisulfide bonded cysteines are eliminated by changing them with another amino acid such as serine or an alanine. A unique cysteine residue is then incorporated into a recombinant protein using site-directed mutagenesis technique, and further reacted with sulfhydryl-specific nitroxide reagent to covalently generate a stable spin label side-chain. The development of SDSL approaches extended the application of EPR to almost any biological systems. Site-directed spin labeling (SDSL) combined with EPR spectroscopy can provide structural dynamics of spin label side-chain, solvent accessibility, solvent polarity, and intra- or intermolecular distances between two spin labels of macromolecules.
- electron paramagnetic resonance (EPR)
- site-directed spin labeling (SDSL)
- membrane protein
1. Introduction
EPR spectroscopy is a magnetic resonance technique that detects materials that contain an unpaired electron. In the presence of an external magnetic field, EPR measures interactions of microwave radiation with the energy splitting of the unpaired electron. EPR spectroscopy works on the principle similar to that of nuclear magnetic resonance (NMR) spectroscopy. The difference is that NMR detects the coupling of NMR-active nuclei of the individual atom with an external magnetic field opposed to the detection of the coupling of unpaired electron with an external magnetic field in the species by EPR. Due to the involvement of the unpaired electron in the spin probe, this technique is very sensitive and can provide up to three orders of magnitude higher sensitivity when compared to nuclear magnetic resonance (NMR) spectroscopic techniques [1]. EPR techniques are complementary to NMR for studying bio-macromolecules. In a continuous wave (CW)-EPR experiment, an external magnetic field is varied at a fixed electromagnetic radiation (microwave) frequency until the EPR transition occurs at the resonance condition when the constant microwave energy matches with the energy associated with the separation between the two electron spin states [2]. The magnetic field is additionally modulated to improve the signal to noise of the EPR signal leading to a derivative lineshape spectrum typically observed in most CW-EPR experiments. Details of theory behind CW- EPR spectroscopic methods can be found in the literature [2][3][4]. EPR spectroscopy can solve several biologically important problems that are very difficult to be studied by conventional biophysical techniques. These include structural and dynamic information for protein systems in solution and membrane bound states [5][6][7][8].
2. Site Directed Spin Labeling (SDSL) Approaches for EPR Spectroscopy
Site-directed spin labeling (SDSL) is a molecular biology approach in which paramagnetic spin labels are incorporated into the specific site of bio-macromolecules. This technique was developed by Hubbell and co-workers more than three decades back [9][10][11]. In the early history, the lack of the unpaired electrons in most biological systems hindered the application of EPR techniques to limited bio-macromolecules such as metalloproteins containing paramagnetic centers and enzymes with radicals. The development of SDSL approaches helped quickly expand the application of EPR to almost any biological systems. In SDSL, all native nondisulfide-bonded cysteines are removed by switching them with another amino acid such as serine or an alanine. A unique cysteine residue is then incorporated into a recombinant protein using site-directed mutagenesis technique, followed by a reaction with sulfhydryl-specific nitroxide reagent to covalently generate a stable spin label side-chain [12][13][14]. Nitroxide spin labels have conformational flexibility with the label scaffold, and the linker between the scaffold and the backbone of the protein. The nitroxide spin labels are kinetically or sterically stabilized by carbon centers in the α-position to the nitrogen atom with alkyl substituents (i.e., methyl, ethyl or higher alkyl substituents) against the reduction of nitroxides [15][16]. Detailed scheme of nitroxide spin labels with different alkyl substituents can be found in the recent literature [16][17][18]. However, larger spin labels still have an increased potential to perturb the structure of the labeled protein. It is critical to optimize the introduction of the nitroxide spin labels during the sample preparation to obtain a stable spin label side chain with minimal structural-functional perturbations on the protein of interest. The most commonly used nitroxide based spin label for studying structural dynamics of membrane proteins is methanethiosulfonate spin label (MTSL). Recently, a more restricted bifunctional spin label (BSL) has been utilized to perform EPR studies of membrane proteins and peptides [19][20][21][22][23]. A chemical structure of MTSL and BSL, and an illustrative example of the reaction of MTSL and BSL with the cysteine residues of the protein and resulting spin label side-chains are shown in Figure 1. Details of nitroxide spin labels utilized for site-directed spin labeling EPR study of bio-macromolecules can be found in the literature [15][24].
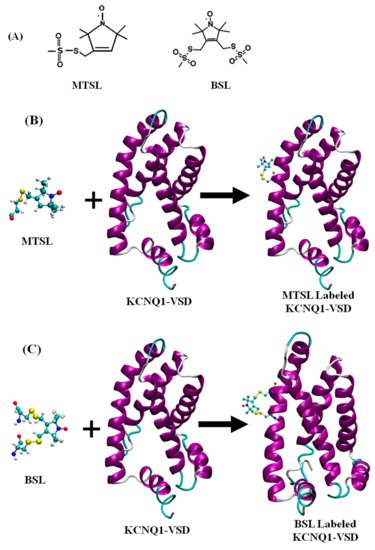
Figure 1. (A) A chemical structure of MTSL (methanethiosulfonate spin label) and BSL (bifunctional spin label). (B) Cartoon representation of the structure of MTSL and the resulting side-chain produced by reaction with a cysteine residue (L134C) and (C) the structure of BSL (bifunctional spin label) and the resulting side-chain produced by reaction with cysteine residues (L134C and I138C) on a KCNQ1-VSD membrane protein. The incorporation of MTSL and BSL spin labels on KCNQ1-VSD (PDB ID:6MIE) was obtained using Charmm-GUI (http://www.charmm-gui.org, accessed on 6 March 2021) [25]. The cartoon structure of the MTSL-labeled and BSL-labeled KCNQ1-VSD was rendered using visual molecular dynamics (VMD) [26].
For typical SDSL experiments on membrane proteins, a 10–20 molar excess of MTSL is mixed with membrane protein samples containing cysteine residue at the site specific location solubilized in the appropriate buffer and pH containing detergent micelles [27]. The reaction is carried out at room temperature or 4 °C for overnight or 24 h depending on the stability of the protein. The non-bonded free spin labels are removed using standard dialysis, or passing through a PD-10 desalting column or size exclusion or ion chromatographic techniques. The spin labeling efficiency is usually determined by comparing protein concentration with the spin label concentration obtained from CW-EPR spectral intensity and analyzing mass spectroscopy data [28]. The optimum spin labeling efficiency is very important to achieve superior EPR data quality.
CW-EPR spectroscopy of spin-labeled macromolecules can provide structural dynamics of nitroxide side-chain, solvent accessibility, solvent polarity, and intra- or intermolecular distances between two nitroxides [5][29][30][12][15][31]. The EPR spectral lineshape analysis of the series of spin-labeled protein sequences can be used to probe the secondary structural information of the protein systems [30][32][33][34][35].
Distance measurements obtained by using double SDSL EPR spectroscopy is very popular and a rapidly growing structural biology technique to probe secondary, tertiary and quaternary structures of bio-macromolecules [30][15][36][37][38]. Distances and distance distribution can be also utilized to obtain conformational rearrangements or complex formation of membrane proteins. The relative orientations between interacting spin labels on the protein can be obtained by using dual SDSL EPR techniques [39][40]. The measurement of magnetic dipolar interactions between two spin labels is analyzed to determine distance between spin labels. The energy of the magnetic dipolar interaction is inversely related to the cube of the distance (r-3) between two spin labels attached on the protein system. The magnetic dipolar interaction significantly broadens the CW-EPR spectral lineshape if the distance is less than 20 Å. The strength of the dipolar interaction is calculated qualitatively from the degree of line broadening using a variety of lineshape analysis techniques to obtain distance information [36][39][41][42][43][44][45].
A distance range of 20–80 Å can be measured by using pulsed double electron electron resonance (DEER) spectroscopy [46]. For DEER experiments, the dipolar coupling between two spins can be measured by observing one set of spins when another set of spins are excited with a second microwave frequency. This leads to a determination of the distance between these two spin labels [46][47][48]. In DEER, intramolecular dipolar interaction modulates the spin echo decay of one set of spin labels with another set of spin labels on the same protein molecule and/or same set of spins or another set of spins on a separate molecule. The oscillating echo periodicity during the former process directly relates the average distance and distance distribution, while later process is an exponential decay which diminishes the oscillation, which is known as the background. The background contribution is removed from the echo decay during the data analysis providing distance distribution accounted by the weighted average distance and a standard deviation. There are several data analysis program freely available including the DeerAnalysis program developed by Jeschke et al. to obtain distance and related information from experimental DEER data [49]. Recently, DEERLab is a new program established for data analysis using Python [50]. A new method based on Deep neural network processing of DEER data has been developed by Worswick et al. and has been incorporated as options into Spinach and DeerAnalysis packages [51].
Nitroxide spin labeling based SDSL DEER spectroscopy is a widely used biophysical technique for studying secondary, tertiary and quaternary structures, and conformational dynamics of a numerous membrane proteins [29][19][52][30][31][46][53][54][55][56][57][58][59]. However, other spin labels including functionalized chelators of paramagnetic lanthanides (GdIII), carbon-based radicals ((trityl), and metals such as copper (CuII) have been recently applied for DEER experiments for studying membrane proteins [60][61][62][63][64][65]. A special care should be taken while choosing specific spin labels and spin labeling sites on membrane proteins because some non-nitroxide spin labels are bulkier such as Gd-based and trityl labels than nitrixide spin labels which can cause perturbation in protein structure and function [60][61][62][63][66]. Earlier studies have suggested that there is no significant perturbation on the structure and/or function of the protein due to nitroxide spin labeling on membrane proteins [29][67][68][69]. However, spin labeling at particular sites of some of membrane proteins may cause significant structural and functional perturbation and poor expression yield, and hence a care should be taken during the experimental design of nitroxide spin labeling sites for double spin labeling experiments.
This entry is adapted from the peer-reviewed paper 10.3390/biophysica1020009
References
- Berliner, L.J. From spin-labeled proteins to in vivo EPR applications. Eur. Biophys. J. 2010, 39, 579–588.
- Weil, J.A.; Bolton, J.R. Electron Paramagnetic Resonance: Elementary Theory and Practical Applications; Wiley-Interscience: Hoboken, NJ, USA, 2007.
- Goldfarb, D.; Stoll, S. EPR Spectroscopy: Fundamentals and Methods; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2018.
- Roessler, M.M.; Salvadori, E. Principles and applications of EPR spectroscopy in the chemical sciences. Chem. Soc. Rev. 2018, 47, 2534–2553.
- Klug, C.S.; Feix, J.B. Methods and Applications of Site-Directed Spin Labeling EPR Spectroscopy. Methods Cell Biol. 2008, 84, 617–658.
- Hubbell, W.L.; Gross, A.; Langen, R.; Lietzow, M.A. Recent advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 1998, 8, 649–656.
- Hubbell, W.L.; Mchaourab, H.S.; Altenbach, C.; Lietzow, M.A. Watching proteins move using site-directed spin labeling. Structure 1996, 4, 779–783.
- Bordignon, E.; Steinhoff, H.-J. Membrane Protein Structure and Dynamics Studied by Site-Directed Spin-Labeling ESR. In ESR Spectroscopy in Membrane Biophysics; Springer: Boston, MA, USA, 2007; Volume 27, pp. 129–164.
- Altenbach, C.; Flitsch, S.L.; Khorana, H.G.; Hubbell, W.L. Structural studies on transmembrane proteins. 2. Spin labeling of bacteriorhodopsin mutants at unique cysteines. Biochemistry 1989, 28, 7806–7812.
- Altenbach, C.; Froncisz, W.; Hyde, J.; Hubbell, W. Conformation of spin-labeled melittin at membrane surfaces investigated by pulse saturation recovery and continuous wave power saturation electron paramagnetic resonance. Biophys. J. 1989, 56, 1183–1191.
- Altenbach, C.; Marti, T.; Khorana, H.G.; Hubbell, W.L. Transmembrane protein structure: Spin labeling of bacteriorhodopsin mutants. Science 1990, 248, 1088–1092.
- Klare, J.P.; Steinhoff, H.-J. Spin labeling EPR. Photosynth. Res. 2009, 102, 377–390.
- Steinhoff, H.-J. Multi-Frequency EPR Spectroscopy Studies of the Structure and Conformational Changes of Site-Directed Spin Labelled Membrane Proteins. In Supramolecular Structure and Function 8; Springer: Boston, MA, USA, 2005; Volume 8, pp. 157–177.
- Cornish, V.W.; Benson, D.R.; Altenbach, C.A.; Hideg, K.; Hubbell, W.L.; Schultz, P.G. Site-specific incorporation of biophysical probes into proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 2910–2914.
- Roser, P.; Schmidt, M.J.; Drescher, M.; Summerer, D. Site-directed spin labeling of proteins for distance measurements in vitro and in cells. Org. Biomol. Chem. 2016, 14, 5468–5476.
- Haugland, M.M.; Lovett, J.E.; Anderson, E.A. Advances in the synthesis of nitroxide radicals for use in biomolecule spin labelling. Chem. Soc. Rev. 2018, 47, 668–680.
- Karthikeyan, G.; Bonucci, A.; Casano, G.; Gerbaud, G.; Abel, S.; Thomé, V.; Kodjabachian, L.; Magalon, A.; Guigliarelli, B.; Belle, V.; et al. A Bioresistant Nitroxide Spin Label for In-Cell EPR Spectroscopy: In Vitro and In Oocytes Protein Structural Dynamics Studies. Angew. Chem. Int. Ed. 2018, 57, 1366–1370.
- Bleicken, S.; Assafa, T.E.; Zhang, H.; Elsner, C.; Ritsch, I.; Pink, M.; Rajca, S.; Jeschke, G.; Rajca, A. Bordignon, E. gem-Diethyl Pyrroline Nitroxide Spin Labels: Synthesis, EPR Characterization, Rotamer Libraries and Biocompatibility. Chemistryopen 2019, 8, 1057–1065.
- Sahu, I.D.; McCarrick, R.M.; Troxel, K.R.; Zhang, R.; Smith, H.J.; Dunagan, M.M.; Swartz, M.S.; Rajan, P.V.; Kroncke, B.M.; Sanders, C.R.; et al. DEER EPR Measurements for Membrane Protein Structures via Bifunctional Spin Labels and Lipodisq Nanoparticles. Biochemistry 2013, 52, 6627–6632.
- Sahu, I.D.; Craig, A.F.; Dunagum, M.M.; McCarrick, R.M.; Lorigan, G.A. Characterization of bifunctional spin labels for inves-tigating the structural and dynamic properties of membrane proteins using EPR spectroscopy. J. Phys. Chem. B 2017, 121, 9185–9195.
- McCaffrey, J.E.; James, Z.M.; Svensson, B.; Binder, B.P.; Thomas, D.D. A bifunctional spin label reports the structural topology of phospholamban in magnetically-aligned bicelles. J. Magn. Reson. 2016, 262, 50–56.
- Li, Q.; Wanderling, S.; Sompornpisut, P.; Perozo, E.; Somponspisut, P. Structural basis of lipid-driven conformational transitions in the KvAP voltage-sensing domain. Nat. Struct. Mol. Biol. 2014, 21, 160–166.
- Lösel, R.M.; Philipp, R.; Kálai, T.; Hideg, K.; Trommer, W.E. Synthesis and Application of Novel Bifunctional Spin Labels. Bioconjug. Chem. 1999, 10, 578–582.
- Haugland, M.M.; Anderson, E.A.; Lovett, J.E. Tuning the properties of nitroxide spin labels for use in electron paramagnetic resonance spectroscopy through chemical modification of the nitroxide framework. In Electron Paramagnetic Resonance; Chechik, V., Murphy, D.M., Eds.; Royal Society of Chemistry (RSC): London, UK, 2017; pp. 1–34.
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. Software news and updates—CHARNIM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865.
- Humphrey, W.; Dalke, A.; Schulten, K. VMD-Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38.
- Sahu, I.D.; Craig, A.F.; Dunagan, M.M.; Troxel, K.R.; Zhang, R.; Meiberg, A.G.; Harmon, C.N.; McCarrick, R.M.; Kroncke, B.M.; Sanders, C.R.; et al. Probing Structural Dynamics and Topology of the KCNE1 Membrane Protein in Lipid Bilayers via Site-Directed Spin Labeling and Electron Paramagnetic Resonance Spectroscopy. Biochemistry 2015, 54, 6402–6412.
- Basak, S.; Chatterjee, S.; Chakrapani, S. Site directed spin labeling and EPR spectroscopic studies of pntameric ligand-gated ion channels. JOVE J. Vis. Exp. 2016, 113, 54127.
- Sahu, I.D.; McCarrick, R.M.; Lorigan, G.A. Use of Electron Paramagnetic Resonance to Solve Biochemical Problems. Biochemistry 2013, 52, 5967–5984.
- Sahu, I.D.; Lorigan, G.A. Electron Paramagnetic Resonance as a Tool for Studying Membrane Proteins. Biomolecules 2020, 10, 763.
- Sahu, I.D.; Lorigan, G.A. Biophysical EPR Studies Applied to Membrane Proteins. J. Phys. Chem. Biophys. 2015, 5, 188.
- Jeschke, G.; Bender, A.; Schweikardt, T.; Panek, G.; Decker, H.; Paulsen, H. Localization of the N-terminal Domain in Light-harvesting Chlorophyll a/b Protein by EPR Measurements. J. Biol. Chem. 2005, 280, 18623–18630.
- Mchaourab, H.S.; Perozo, E. Determination of Protein Folds and Conformational Dynamics Using Spin-Labeling EPR Spec-troscopy. In Biological Magnetic Resonance; Berliner, L., Eaton, G., Eaton, S., Eds.; Springer: New York, NY, USA, 2002; pp. 185–247.
- Perozo, E.; Cortes, D.M.; Cuello, L.G. Three-dimensional architecture and gating mechanism of a K+ channel studied by EPR spectroscopy. Nat. Struct. Biol. 1998, 5, 459–469.
- Vasquez, V.; Sotomayor, M.; Cortes, D.M.; Roux, B.; Schulten, K.; Perozo, E. Three-dimensional architecture of membrane-embedded MscS in the closed conformation. J. Mol. Biol. 2008, 378, 55–70.
- Hustedt, E.J.; Beth, A.H. Nitroxide spin-spin interactions: Applications to protein structure and dynamics. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 129–153.
- Brown, L.J.; Hare, J.E. Electron Paramagnetic Resonance: Site-Directed Spin Labeling; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015.
- Wunnicke, D.; Hänelt, I. The Synergetic Effects of Combining Structural Biology and EPR Spectroscopy on Membrane Proteins. Crystals 2017, 7, 117.
- Hustedt, E.; Smirnov, A.; Laub, C.; Cobb, C.; Beth, A. Molecular distances from dipolar coupled spin-labels: The global analysis of multifrequency continuous wave electron paramagnetic resonance data. Biophys. J. 1997, 72, 1861–1877.
- Ghimire, H.; Hustedt, E.J.; Sahu, I.D.; Inbaraj, J.J.; McCarrick, R.; Mayo, D.J.; Benedikt, M.R.; Lee, R.T.; Grosser, S.M.; Lorigan, G.A. Distance Measurements on a Dual-Labeled TOAC AChR M2δ Peptide in Mechanically Aligned DMPC Bilayers via Dipolar Broadening CW-EPR Spectroscopy. J. Phys. Chem. B 2012, 116, 3866–3873.
- Hustedt, E.J.; Stein, R.A.; Sethaphong, L.; Brandon, S.; Zhou, Z.; DeSensi, S.C. Dipolar Coupling between Nitroxide Spin Labels: The Development and Application of a Tether-in-a-Cone Model. Biophys. J. 2006, 90, 340–356.
- Banham, J.E.; Baker, C.M.; Ceola, S.; Day, I.J.; Grant, G.H.; Groenen, E.J.; Rodgers, C.T.; Jeschke, G.; Timmel, C.R. Distance measurements in the borderline region of applicability of CW EPR and DEER: A model study on a homologous series of spin-labelled peptides. J. Magn. Reson. 2008, 191, 202–218.
- Rabenstein, M.D.; Shin, Y.K. Determination of the distance between two spin labels attached to a macromolecule. Proc. Natl. Acad. Sci. USA 1995, 92, 8239–8243.
- Czogalla, A.; Pieciul, A.; Jezierski, A.; Sikorski, A.F. Attaching a spin to a protein—Site-directed spin labeling in structural biology. Acta Biochim. Pol. 2007, 54, 235–244.
- Mandal, T.; Hustedt, E.J.; Song, L.; Oh, K.J. CW EPR and DEER Methods to Determine BCL-2 Family Protein Structure and Interactions: Application of Site-Directed Spin Labeling to BAK Apoptotic Pores. In BCL-2 Family Proteins; Methods in Molecular Biology; Gavathiotis, E., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1877, pp. 257–303.
- Jeschke, G.; Polyhach, Y. Distance measurements on spin-labelled biomacromolecules by pulsed electron paramagnetic resonance. Phys. Chem. Chem. Phys. 2007, 9, 1895–1910.
- Schweiger, A.; Jeschke, G. Principles of Pulse Electron Paramagnetic Resonance; Oxford University Press: New York, NY, USA, 2001.
- Pannier, M.; Veit, S.; Godt, A.; Jeschke, G.; Spiess, H.W. Dead-time free measurement of dipole-dipole interactions between electron spins. J. Magn. Reson. 2000, 142, 331–340.
- Jeschke, G.; Chechik, V.; Ionita, P.; Godt, A.; Zimmermann, H.; Banham, J.; Timmel, C.R.; Hilger, D.; Jung, H. Deer Analysis 2006—A comprehensive software package for analyzing pulsed ELDOR data. Appl. Magn. Reson. 2006, 30, 473–498.
- Ibáñez, L.F.; Jeschke, G.; Stoll, S. DeerLab: A comprehensive software package for analyzing dipolar electron paramagnetic resonance spectroscopy data. Magn. Reson. 2020, 1, 209–224.
- Worswick, S.G.; Spencer, J.A.; Jeschke, G.; Kuprov, I. Deep neural network processing of DEER data. Sci. Adv. 2018, 4, eaat5218.
- Sahu, I.D.; Kroncke, B.M.; Zhang, R.; Dunagan, M.M.; Smith, H.J.; Craig, A.; McCarrick, R.M.; Sanders, C.R.; Lorigan, G.A. Structural Investigation of the Transmembrane Domain of KCNE1 in Proteoliposomes. Biochemistry 2014, 53, 6392–6401.
- Sahu, I.D.; Lorigan, G.A. Site-Directed Spin Labeling EPR for Studying Membrane Proteins. BioMed Res. Int. 2018, 2018, 3248289.
- Sahu, I.D.; Lorigan, G.A. EPR Techniques, Spin Labeling and Spin Trapping. In Encyclopedia of Analytical Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 315–327.
- Borbat, P.P.; McHaourab, H.S.; Freed, J.H. Protein structure determination using long-distance constraints from double-quantum coherence ESR: Study of T4 lysozyme. J. Am. Chem. Soc. 2002, 124, 5304–5314.
- Jeschke, G. DEER Distance Measurements on Proteins. Annu. Rev. Phys. Chem. 2012, 63, 419–446.
- Milov, A.D.; Tsvetkov, Y.D.; Formaggio, F.; Crisma, M.; Toniolo, C.; Raap, J. Self-assembling properties of membrane-modifying peptides studied by PELDOR and CW-ESR spectroscopies. J. Am. Chem. Soc. 2000, 122, 3843–3848.
- Hilger, D.; Jung, H.; Padan, E.; Wegener, C.; Vogel, K.P.; Steinhoff, H.J.; Jeschke, G. Assessing oligomerization of membrane proteins by four-pulse DEER: pH-dependent dimerization of NhaA Na+/H+ antiporter of E. coli. Biophys. J. 2005, 89, 1328–1338.
- Ahammad, T.A.; Drew, D.L.; Sahu, I.D.; Khan, R.H.; Butcher, B.J.; Serafin, R.A.; Galende, A.P.; McCarrick, R.M.; Lorigan, G.A. Conformational Differences are Observed for the Active and Inactive Forms of Pinholin S21 using DEER Spectroscopy. Phys. Chem. B 2020, 124, 11396–11405.
- Bordignon, E.; Bleicken, S. New limits of sensitivity of site-directed spin labeling electron paramagnetic resonance for membrane proteins. Biochim. Biophys. Acta Biomembr. 2018, 1860, 841–853.
- Feintuch, A.; Otting, G.; Goldfarb, D. Gd3+ Spin Labeling for Measuring Distances in Biomacromolecules: Why and How? In Methods in Enzymology; Electron Paramagnetic Resonance Investigations of Biological Systems by Using Spin Labels, Spin Probes, and Intrinsic Metal Ions, Part A; Elsevier: Amsterdam, The Netherlands, 2015; Volume 563, pp. 415–457.
- Jassoy, J.J.; Berndhäuser, A.; Duthie, F.; Kühn, S.P.; Hagelueken, G.; Schiemann, O. Versatile Trityl Spin Labels for Nanometer Distance Measurements on Biomolecules In Vitro and within Cells. Angew. Chem. Int. Ed. 2017, 56, 177–181.
- Yang, Z.Y.; Ji, M.; Cunningham, T.F.; Saxena, S. Cu2+ as an ESR Probe of Protein Structure and Function. In Methods in Enzymology; Electron Paramagnetic Resonance Investigations of Biological Systems by Using Spin Labels, Spin Probes, and Intrinsic Metal Ions, Part A; Elsevier: Amsterdam, The Netherlands, 2015; Volume 563, pp. 459–481.
- Joseph, B.; Sikora, A.; Cafiso, D.S. Ligand Induced Conformational Changes of a Membrane Transporter in E. coli Cells Observed with DEER/PELDOR. J. Am. Chem. Soc. 2016, 138, 1844–1847.
- Yardeni, E.H.; Bahrenberg, T.; Stein, R.A.; Mishra, S.; Zomot, E.; Graham, B.; Tuck, K.L.; Huber, T.; Bibi, E.; McHaourab, H.S.; et al. Probing the solution structure of the E. coli multidrug transporter MdfA using DEER distance measurements with nitroxide and Gd(III) spin labels. Sci. Rep. 2019, 9, 12528.
- Joseph, B.; Tormyshev, V.M.; Rogozhnikova, O.Y.; Akhmetzyanov, D.; Bagryanskaya, E.G.; Prisner, T.F. Selective High-Resolution Detection of Membrane Protein-Ligand Interaction in Native Membranes Using Trityl-Nitroxide PELDOR. Angew. Chem. Int. Ed. 2016, 55, 11538–11542.
- Altenbach, C.; Yang, K.; Farrens, D.L.; Farahbakhsh, Z.T.; Khorana, H.G.; Hubbell, W.L. Structural features and light-dependent changes in the cytoplasmic interhelical E-F loop region of rhodopsin: A site-directed spin-labeling study. Biochemistry 1996, 35, 12470–12478.
- Hubbell, W.L.; Altenbach, C. Investigation of structure and dynamics in membrane proteins using site-directed spin labeling. Curr. Opin. Struct. Biol. 1994, 4, 566–573.
- Fajer, P.G. Site directed spin labelling and pulsed dipolar electron paramagnetic resoonance (double electron-electron resonance) of force activation in muscle. J. Phys. Condens. Matter 2005, 17, S1459–S1469.
