Aparna Das*a, Ajay K. Boseb and Bimal Krishna Banik*c
aDepartment of Mathematics and Natural Sciences, College of Sciences and Human Studies, Prince Mohammad Bin Fahd University, Al Khobar 31952, KSA; Email: aparnadasam@gmail.com; Email: adas@pmu.edu.sa
bDepartment of Chemistry and Chemical Biology, Stevens Institute of Technology, Hoboken, New Jersey 07030, USA;
cDepartment of Mathematics and Natural Sciences, College of Sciences and Human Studies, Deanship of Research, Prince Mohammad Bin Fahd University, Al Khobar 31952, KSA; Email: bimalbanik10@gmail.com; bbanik@pmu.edu.sa
Abstract:
Stereoselective synthesis of cis and trans β-lactams under diverse conditions is performed. Numerous conditions are used for this study. The formation of β-lactam depends on the conditions of the experiments, structures of the imines and acid chlorides, order of addition of the reagents, reaction temperature, and solvents. A few mathematical graphs are plotted to explain the results.
- β-Lactam
- Stereoselectivity
- Microwave
INTRODUCTION
β-Lactams are medicinally active molecules. Several publications have disclosed the anticancer [1], antibacterial [2], antifungal [3], cholesterol absorption inhibitors [4], anti-inflammatory [5], anti-hepatitis [6], analgesic activities [7] and antihyperglycemic [8] properties of β-lactams. Many methods are available for the preparation of β-lactams, such as Staudinger cycloaddition [9], hydroxamate approach [10], ester enolate-imine condensation [11], alkene-isocyanate method [12], the alkyne-nitrone reaction (Kinugasa reaction) [13], catalytic asymmetric synthesis [14] and polymer-supported method [15]. Our group has also demonstrated the synthesis of β-lactams [16].
Depending on the reactants and reaction conditions the stereochemistry of β-lactams may alter. Both the stereoisomers are important for medicinal applications. Thus, controlling diastereoselectivity (cis or trans) of the β-lactams is important. Stereoselective synthesis of diverse β-lactams following a variety of conditions through cycloaddition reaction of imines and acid chlorides is described. The results are also explained by plotting the ratios of the two isomeric β-lactams formed with respect to the time of the reaction.
RESULTS WITH ACETOXY DERIVATIVE
The Staudinger reaction mainly required an imine, a tertiary base, and an acid chloride. The reaction of an acid chloride or equivalent with an imine in the presence of a tertiary produced cis and trans isomers of β-lactams. In this study, ten different reaction conditions including microwave-induced organic reaction enhancement (MORE) chemistry techniques and traditional synthesis/one-pot synthesis was adopted. A domestic microwave oven was used for irradiation and a large Erlenmeyer flask was used as the reaction vessel.
Experiment 1:
Microwave irradiation of a solution of imine with an acid chloride in chlorobenzene [17] produced a mixture of cis and trans β-lactams. N-methylmorpholine (NMM) was used as a base for this reaction instead of trimethylamine (TEA). Non-polar solvent, benzene was chosen as the reaction medium and reaction temperature was kept in between 45°C-50°C. It was observed that the reaction is not completed after 4 min and it produced a mixture of cis (70%) and trans (30%) β-lactams. The ratios of the cis and trans-isomers were determined from the coupling constants of the C3 and C4 protons of the β-lactam rings.
Experiment 2:
To identify the effect of the polarity of the solvent on stereoselectivity, chlorobenzene was used. Chlorobenzene being a polar solvent absorbs microwave energy efficiently. NMM was chosen as a base and reaction temperature was between 95°C-100°C. The reaction was completed within 5 min and it produced a mixture of cis (5-10%) and trans (90-95%). Thus this reaction condition was suitable for the preparation of trans β-lactams.
Experiment 3:
The third reaction was undertaken without any solvent. The reaction between the imine and acid chloride was conducted in a microwave oven at the temperature range of 95°C-100°C in the presence of NMM. The temperature was noted when the reaction was performed with 10 mmol of the substrates. The reaction produced a mixture of cis (5-10%) and trans (90-95%) isomers. It appeared that the solvent makes the reaction slower.
Experiment 4:
The reaction was conducted in a preheated oil bath at 900C in the presence of NMM. The reaction was completed within 5 min and it gave a mixture of cis (5-10%) and trans (90-95%) β-lactams.
Experiment 5:
In another experiment, the oil bath was used, but the temperature was gradually increased from room temperature to 900C. Chlorobenzene was chosen as the solvent and NMM was the base. The same reaction of the imine with acid chloride was performed and it was completed within 15 min. It produced a mixture of cis (50%) and trans (50%) β-lactams.
Experiment 6:
Another variation of this reaction was conducted using a one-pot method. In this experiment, benzaldehyde and p-anisidine were reacted in the presence of clay. NMM, AcOCH2COCl, and chlorobenzene were added to it. Irradiating the reaction mixture in a microwave for 2 min, the trans isomer of β-lactam was formed. The reaction produced only the cis isomer in the absence of microwave irradiation at room temperature.
Results with Acetoxy Derivative:
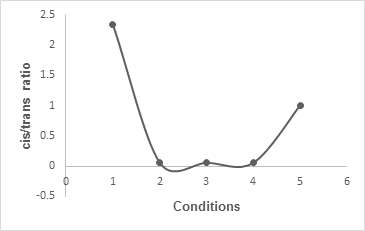
The results obtained under different reaction conditions were extremely interesting. Table 1 showed the ratios of the cis and trans β-lactams obtained under different conditions. The data showed that reaction conditions 2, 3, 4, and 6(a) were helpful for the synthesis of trans β-lactam. In contrast, reaction conditions 1 and 6(b) were good for the synthesis of cis β-lactam. On the other hand, reaction condition 5 was perfect for the synthesis of a mixture of cis and trans β-lactams. The graphical representation of these observations under diverse conditions is shown in Figure 1.
|
Experiments |
Reaction temperature |
Time |
cis/trans ratio |
|
1 |
45°C-50°C |
4 min |
70:30 |
|
2 |
95°C-100°C |
5 min |
5:95 |
|
3 |
95°C-100°C |
3 min |
5:95 |
|
4 |
90°C |
5 min |
5:95 |
|
5 |
RT-90°C |
15 min |
50:50 |
|
6 (a)† |
95°C-100°C |
5-10 min |
0:100 |
|
6 (b) † |
0°C-RT |
Overnight |
100:0 |
Table 1: Ratios of the cis and trans lactams under diverse conditions.
Figure 1: Graphical representation of the β-lactam formation under diverse conditions.
Also, the cis β-lactams did not change to trans β-lactams when they were treated with NMM in chlorobenzene in a domestic microwave oven for 2-3 min even at 900C. This experiment established that there is no isomerization of the cis β-lactams to the more thermodynamically stable trans β-lactams under microwave irradiation at a high temperature.
The reaction was performed with phenyl-substituted imine and similar ratios of the cis and trans isomers were formed.
Experiment 7:
The reaction of benzyloxyacetyl chloride with imine in the presence of dimethyl-formamide (DMF) and NMM also yielded a mixture of cis and trans-β-lactam in varying proportions.
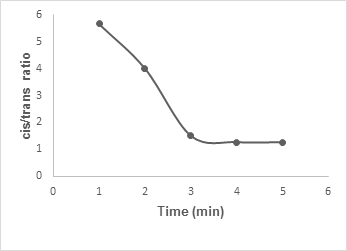
Table 2 showed the cis/trans ratios during the time of microwave irradiation. The results showed that cis lactams were formed with low power radiation. On the other hand, high power radiation generated trans lactams formation preferentially.
|
Time |
Temperature |
Power |
Cis |
Trans |
cis/trans ratio |
|
1 min |
70°C |
Low |
85 |
15 |
85:15 |
|
2 min |
75°C |
Low |
80 |
20 |
80:20 |
|
3 min |
80°C |
Low |
60 |
40 |
60:40 |
|
4 min |
95°C |
Low |
56 |
44 |
56:44 |
|
5 min |
97°C |
Low |
55 |
45 |
55:45 |
|
4 min |
110°C |
High |
45 |
55 |
45:55 |
Table 2: Ratios of the cis and trans lactams with respect to time.
Figure 2 represented graphically the variation of the cis and trans ratios with irradiation time up to 5 min at low power mode. The data indicated that the cis/trans ratio decreases with the progress of time and finally stabilizes at 4-5 min.
Figure 2: Graphical representation of the cis/trans ratio of β-lactam with the time of irradiation.
Experiment 8:
Microwave irradiation of activated phthalimido acetic acid with imine in the presence of chlorobenzene and NMM produced a mixture of cis and trans β-lactams.
Experiment 9:
Microwave irradiation of acid chloride with imine (produced from D-glyceraldehyde) in the presence of chlorobenzene and NMM in 0-5 min gave cis β-lactam. However, irradiation of acid chloride with imine (obtained from L-glyceraldehyde) gave cis β-lactam with opposite absolute stereochemistry.
Experiment 10:
trans β-Lactam was formed in 100% yield by slow addition of NMM in ethylene dichloride to a refluxing solution of imine and the acid chloride.
CONCLUSIONS
The stereochemistry (cis and/or trans) of the β-lactams under diverse conditions was analyzed. The data showed that some reaction conditions are favorable for the synthesis of trans β-lactams. On the other hand, some reaction conditions are favorable for the synthesis of cis β-lactams. A few reaction conditions are favorable for the synthesis of a mixture of cis and trans β-lactam. The diastereoselectivity of the β-lactam formation strongly depends on reactants and reaction conditions. The data suggest that the β-lactam formation reaction depends on two pathways. One pathway is favored at high temperatures and or concentrated solution/microwave-mediated reaction conditions.
CONFLICT OF INTEREST
The authors confirm that this result has no conflict of interest.
ACKNOWLEDGEMENTS
AD and BKB are grateful to Prince Mohammad Bin Fahd University for support. BKB is also grateful to US NIH, US NCI, and Kleberg Foundation of Texas for finance support.
REFERENCES
- (a) Banik, I; Becker, F.F.; Banik, B.K. Stereoselective Synthesis of β-Lactams with Polyaromaic Imines: Entry to New and Novel Anticancer Agents. J. Med. Chem., 2003, 46, 12-15; (b) Banik, B.K.; Becker, F.F.; Banik, I. Synthesis of Anticancer β-Lactams: Mechanism of Action. Bioorg. Med. Chem., 2004, 12, 2523-2528; (c) Banik, B.K. Ed. β-Lactams: Synthesis, Stereochemistry, Synthons and Biological Evaluation. Curr. Med. Chem., 2004, Volume 12; (d) Banik, B.K.; Banik, I.; Becker, F.F. Stereocontrolled Synthesis of Anticancer β-Lactams via the Staudinger Reaction. Bioorg. Med. Chem., 2005, 13, 3611-3622; (e) Banik, B.K.; Becker, F.F. Selective Anticancer Activity of β-Lactams Derived from Polyaromatic Compound. Mol. Med. Rep., 2010, 3, 315-316; (f) Banik, B.K.; Banik, I.; Becker, F.F. Asymmetric Synthesis of Anticancer β-Lactams via Staudinger Reaction: Utilization of Chiral Ketene from Carbohydrate. Eur. J. Med. Chem., 2010, 45, 846-848; (g) Banik, B. K. Curing Cancer Through Manipulation of Molecules. International Innovation; 2011, 50-53; (h) Banik, B. K. Curious Science: Ringing the Changes for Cancer. International Innovation; 2012, 114-116; (i) Banik, B. K.; Samajdar, S.; Becker, F. F. Asymmetric Synthesis of Anticancer β-Lactams Via Staudinger Reaction. Molecular Medicine Reports, 2010, 3, 319-321; (j) Bandyopadhyay, D.; Cruz, J.; Banik, B. K.; Microwave-Induced Synthesis of 3-Pyrrole Substituted β-Lactams Via Bismuth Nitrate-Catalyzed Reactions. Tetrahedron Symposium-in-Print, 2012, 68, 10686-10695; (k) Bandyopadhyay, D.; Rhodes, E.; Banik, B. K. A Green, Chemoselective, and Practical Approach Toward N-(2-azetidinonyl)-2,5-disubstituted Pyrroles. Royal Society Advance, 2013, 3, 16756-16764.
- Sperka, T.; Pitlik, J.; Bagossi, P.; Tözsér J. Beta-lactam compounds as apparently uncompetitive inhibitors of HIV-1 protease. Med. Chem. Lett., 2005, 15,3086.
- O’Driscoll, M.; Greenhalgh, K.; Young, A.;Turos, E.; Dickey, S.; Lim, D.V. Studies on the antifungal properties of N-thiolated beta-lactams. Bioorg. Chem., 2008, 16(16), 7832.
- (a) Clader, J.W.; Burnett, D.A.; Caplen, M.A.; Domalski, M.S.; Dugar, S.; Vaccaro, W.; Sher, R.; Browne, M. E.; Zhao, H.; Burrier, R. E.; Salisbury, B.; Davis, H. R. 2-Azetidinone Cholesterol Absorption Inhibitors: Structure−Activity Relationships on the Heterocyclic Nucleus. Med.Chem., 1996, 39, 3684-3693; (b) Burnett, D. A.; Caplen, M. A.; Darris, H. R., Jr.; Burrier,R. E.; Clader, J. W. 2-Azetidinones as inhibitors of cholesterol absorption. J. Med. Chem., 1994, 37, 1734-1736; (c) Burnett, D.A. Beta-lactam cholesterol absorption inhibitors. Curr. Med. Chem., 2004, 11, 1873–1887; (d) Clader, J.W. The Discovery of Ezetimibe: A View from Outside the Receptor. J. Med. Chem., 2004, 47, 1–9.
- Srivastava, S.K.; Srivastava, S.L.; Srivastava, S.D. Synthesis of new 2-chloro-phenothiazinothiadiazol-2-oxoaze tidines: Antimicrobial and antiinflammatory agents. Indian J. Chem., 2000, 39B, 464.
- Lall, M.S.; Ramtohul, Y.K.; James, M.N.; Vederas, J.C. Serine and Threonine β-Lactones: A New Class of Hepatitis A Virus 3C Cysteine Proteinase Inhibitors. Org. Chem., 2002, 67, 1536.
- Saturnino, C,; Fusco, B.; Saturnino, P.; De Martino, G.; Rocco, F.; Lancelot, C. Evaluation of analgesic and anti-inflammatory activity of novel beta-lactam monocyclic compounds. Biol. Pharm. Bull., 2000, 23, 654.
- Goel, R.K.; Mahajan, M.P.; Kulkarni, S.K. Evaluation of anti-hyperglycemic activity of some novel monocyclic beta lactams. Pharm. Pharm. Sci., 2004, 7, 80.
- (a) Staudinger, H. Zur Kenntniss der Ketene. Diphenylketen. Justus Liebigs Ann. Chem., 1907, 356, 51; (b) Georg, G. I.; Ravikumar, V. T. The Organic Chemistry of b-Lactams; VCH: NewYork, NY,1993, 295; (c) Palomo, C.; Aizpurua, J. M.; Ganboa, I.; Oiarbide, M. Asymmetric Synthesis of β‐Lactams by Staudinger Ketene‐Imine Cycloaddition Reaction. J. Org. Chem., 1999, 12, 3223; (d) Qing, Y.; Jian, S. Z.;Wang, Y. G. Enantioselective Synthesis of β-Lactams Using a Chiral Auxiliary. Synlett, 2006, 1113; (e) Duguet, N.; Donaldson, A.; Leckie, S. M.; Douglas, J.; Shapland, P.; Brown, T. B.; Churchill, G.; Slawin, A. M. Z.; Smith, A. D. Chiral relay in NHC-mediated asymmetric β-lactam synthesis I; substituent effects in NHCs derived from (1R,2R)-cyclohexane-1,2-diamine. Tetrahedron: Asymmetry, 2010, 21, 582; (f) Jarrahpour, A.; Zarei, M. Efficient one-pot synthesis of 2-azetidinones from acetic acid derivatives and imines using methoxymethylene-N,N-dimethyliminium salt. Tetrahedron, 2010, 66, 5017.
- Miller, M. Hydroxamate approach to the synthesis of .beta.-lactam antibiotics. Acc. Chem. Res., 1986, 19, 49.
- (a) Hart, D. J.; Ha, D. C. The ester enolate-imine condensation route to .beta.-lactams. Rev., 1989, 89, 1447; (b) Georg, G. I. In Natural Product Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterderm, 1989, Vol. 4, p 431; (c) Cainelli, G.; Panunzio, M.; Andreoli, P.; Martelli, G.; Spunta, G.; Giacomini, D.; Bandini, E. Metallo-imines: Useful reagents in organic synthesis. Pure Appl. Chem., 1990, 62, 605; (d) Benaglia, M.; Cinquini, M.; Cozzi, F. The S‐Thioester Enolate/Imine Condensation: A Shortcut to β‐Lactams. Eur. J. Org. Chem., 2000, 4, 563.
- Chmielewski, M.; Kaiuza, Z.; Furman, B. Stereocontrolled synthesis of 1-oxabicyclic β-lactam antibiotics via[2 + 2]cycloaddition of isocyanates to sugar vinyl ethers. Commun., 1996, 24, 2689. Also, see Ref. 11h.
- (a) Kinugasa, M.; Hashimoto, S. The reactions of copper(I) phenylacetylide with nitrones. Chem. Soc., Chem. Commun., 1972, 466; (b) Miura, M.; Enna, M.; Okuro, K.; Nomura, M. Copper-Catalyzed Reaction of Terminal Alkynes with Nitrones. Selective Synthesis of 1-Aza-1-buten-3-yne and 2-Azetidinone Derivatives. J. Org. Chem., 1995, 60, 4999; (c) Lo, M. M. C.; Fu, G. C. Cu(I)/Bis(azaferrocene)-Catalyzed Enantioselective Synthesis of β-Lactams via Couplings of Alkynes with Nitrones. J. Am. Chem. Soc., 2002, 124, 4572; (d) Basak, A.; Ghosh, S. C.; Bhowmich, T.; Das, A. K.; Bertolasi, V. An asymmetric synthesis of β-lactams: on the use of chiral oxazolidones in the Kinugasa reaction. Tetrahedron Lett., 2002, 43, 5499; (e) Shintani, R.; Fu, G. C. Catalytic enantioselective synthesis of beta-lactams: intramolecular Kinugasa reactions and interception of an intermediate in the reaction cascade. Angew. Chem., 2003, 42, 4082; (f) Ye, M. C.; Zhou, J.; Tang, Y. Trisoxazoline/Cu(II)-Promoted Kinugasa Reaction. Enantioselective Synthesis of β-Lactams. J. Org. Chem., 2006, 71, 3576.
- (a) Taggi, A. E.; Hafez, A. M.;Wack, H.; Young, B.; Drury,W. J., III; Lectka, T. Catalytic, Asymmetric Synthesis of β-Lactams. Am. Chem. Soc., 2000, 122, 7831; (b) Magriotis, P. A. Neues über die enantioselektive Synthese von β‐Lactamen: Entwicklung der ersten katalytischen Ansätze. Angew. Chem., 2001, 113, 4507; (c) Hodous, B. L.; Fu, G. C. Enantioselective Staudinger Synthesis of β-Lactams Catalyzed by a Planar-Chiral Nucleophile. J. Am. Chem. Soc., 2002, 124, 1578; (d) Cordova, A.; Watanabe, S.; Tanaka, F.; Notz, W.; Barbas, C. F. A Highly Enantioselective Route to Either Enantiomer of Both α- and β-Amino Acid Derivatives. J. Am. Chem. Soc., 2002, 124, 1866; (e) Wack, H.; France, S.; Hafez, A. M.; Drury, W. J.; Weatherwax, A.; Lectka, T. Development of a New Dimeric Cyclophane Ligand: Application to Enhanced Diastereo- and Enantioselectivity in the Catalytic Synthesis of β-Lactams. J. Org. Chem., 2004, 69, 4531; (f) Stefan, F.;Weatherwax, A.; Taggi, A. E.; Lectka, T. Advances in the Catalytic, Asymmetric Synthesis of β-Lactams. Acc. Chem. Res., 2004, 37, 592; (g) Fu, G. C. Asymmetric Catalysis with “Planar-Chiral” Derivatives of 4-(Dimethylamino)pyridine. Acc. Chem. Res., 2004, 37, 542.
- (a) Donati, D.; Morelli, C.; Porcheddu, A.; Taddei, M. A New Polymer-Supported Reagent for the Synthesis of β-Lactams in Solution. Org. Chem., 2004, 69, 9316; (b) Mandal, N.; Ghosh, P.; Basu, B. Recent approaches toward solid phase synthesis of β-lactams. Top. Heterocycl. Chem., 2010, 22, 261.
- (a) Banik, B.K.; Ed. Heterocyclic Scaffolds I. Top. Chem., Springer, 2010, 22, 1-379; (b) Banik, B.K. Ed. β-Lactams: Synthesis and Biological Evaluation, Top. Heterocycl. Chem., Springer, 2012, 30, 1-226; (c) Banik, I.; Banik, B. K. Microwave-Induced Chemical Manipulation of β-Lactam. Springer; 2012, 88, 781-1007; (d) Banik, B.K. Beta Lactams: Novel Synthetic Pathways and Applications. Ed. Springer, 2017, 1-419; (e) Parvatkar, P. T.; Parameswaran, P. S.; Banik, B. K. Solid Phase Synthesis of β-Lactams: Results and Scope in Banik, B. K., Beta Lactams: Novel Synthetic Pathways and Applications, Ed. Spinger, 2017, 253-284; (f) Basu, S.; Banik, B. K. Beta Lactams as Clinically Active Molecules in Banik, B. K., Beta Lactams: Novel Synthetic Pathways and Applications, Ed. Springer, 2017, 285-310; (g) Banik, B. K., Synthesis and Biological Studies of Novel β-Lactams, CRC Book, 2013, 31-72. (h) Ghatak, A.; Becker, F. F.; Banik, B. K. Indium-mediated Facile Synthesis of 3-Unsubstituted Ferrocenyl β-Lactams. Heterocycles, 2000, 53, 2769–2773; (i) Banik, B. K.; Ghatak, A.; Becker, F. F. Indium-mediated facile synthesis of 3-unsubstituted β-lactams. J. Chem. Soc., Perkin Trans., 2000, 1, 2179–2181.
- (a) Perreux, L.; Loupy, A. A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron, 2001, 57, 9199–9223; (b) Lidstrom, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave Assisted Organic Synthesis—A Review. Tetrahedron, 2001, 57, 9225–9283; (c) Bose, A. K.; Manhas, M. S.; Ganguly, S. N.; Sharma, A. H.; Banik, B. K. MORE Chemistry for Less Pollution: Applications for Process Development. Synthesis, 2002, 1578–1591 and references cited therein.
References
- Indrani Banik; Frederick F. Becker; Bimal K. Banik; Stereoselective Synthesis of β-Lactams with Polyaromatic Imines: Entry to New and Novel Anticancer Agents†. Journal of Medicinal Chemistry 2003, 46, 12-15, 10.1021/jm0255825.
- Tamás Sperka; János Pitlik; Péter Bagossi; József Tőzsér; Beta-lactam compounds as apparently uncompetitive inhibitors of HIV-1 protease. Bioorganic & Medicinal Chemistry Letters 2005, 15, 3086-3090, 10.1016/j.bmcl.2005.04.020.
- Marci O’Driscoll; Kerriann Greenhalgh; Ashley Young; Edward Turos; Sonja Dickey; Daniel V. Lim; Studies on the antifungal properties of N-thiolated β-lactams. Bioorganic & Medicinal Chemistry 2008, 16, 7832-7, 10.1016/j.bmc.2008.06.035.
- Duane A. Burnett; Mary Ann Caplen; Harry R. Davis; Robert E. Burrier; John W. Clader; 2-Azetidinones as Inhibitors of Cholesterol Absorption. Journal of Medicinal Chemistry 1994, 37, 1733-1736, 10.1021/jm00038a001.
- Synthesis of new 2-chloro-phenothiazinothiadiazol-2-oxoaze tidines: Antimicrobial and antiinflammatory agents . Indian J. Chem., 2000, 39B, 464. . Retrieved 2020-6-7
- Manjinder S. Lall; Yeeman K. Ramtohul; Michael N. G. James; John C. Vederas; Serine and Threonine β-Lactones: A New Class of Hepatitis A Virus 3C Cysteine Proteinase Inhibitors. The Journal of Organic Chemistry 2002, 67, 1536-1547, 10.1021/jo0109016.
- Carmela Saturnino; Bruno Fusco; Paola Saturnino; Giovanni De Martino; Flavio Rocco; Jean-Charles Lancelot; Evaluation of analgesic and anti-inflammatory activity of novel beta-lactam monocyclic compounds.. Biological & Pharmaceutical Bulletin 2000, 23, 654-656, 10.1248/bpb.23.654.
- Rajesh Kumar Goel; Mohinder P Mahajan; Shrinivas K Kulkarni; Evaluation of anti-hyperglycemic activity of some novel monocyclic beta lactams.. Journal of Pharmacy & Pharmaceutical Sciences 2004, 7, 80, .
- Hermann Staudinger; Zur Kenntniss der Ketene. Diphenylketen. European Journal of Organic Chemistry 1907, 356, 51-123, 10.1002/jlac.19073560106.
- Marvin J. Miller; Hydroxamate approach to the synthesis of .beta.-lactam antibiotics. Accounts of Chemical Research 1986, 19, 49-56, 10.1021/ar00122a004.
- David J. Hart; Deok Chan Ha; The ester enolate-imine condensation route to .beta.-lactams. Chemical Reviews 1989, 89, 1447-1465, 10.1021/cr00097a003.
- Marek Chmielewski; Zbigniew Kałuża; Bartłomiej Furman; Bart?omiej Furman; Stereocontrolled synthesis of 1-oxabicyclic β-lactam antibiotics via[2 + 2]cycloaddition of isocyanates to sugar vinyl ethers. Chemical Communications 1996, 24, 2689-2696, 10.1039/cc9960002689.
- Manabu Kinugasa; Shizunobu Hashimoto; The reactions of copper(I) phenylacetylide with nitrones. J. Chem. Soc., Chem. Commun. 1972, , , 10.1039/c39720000466.
- Andrew E. Taggi; Ahmed M. Hafez; Harald Wack; Brandon Young; William J. Drury; Thomas Lectka; Catalytic, Asymmetric Synthesis of β-Lactams. Journal of the American Chemical Society 2000, 122, 7831-7832, 10.1021/ja001754g.
- Donato Donati; Costanza Morelli; Andrea Porcheddu; Maurizio Taddei; A New Polymer-Supported Reagent for the Synthesis of β-Lactams in Solution. The Journal of Organic Chemistry 2004, 69, 9316-9318, 10.1021/jo048400i.
- Banik, Bimal K. Beta Lactams: Novel Synthetic Pathways and Applications; Banik, Bimal K, Eds.; Springer: Switzerland, 2017; pp. 1-419.