Glaucoma is a heterogeneous group of chronic neurodegenerative disorders characterized by a relatively selective, progressive damage to the retinal ganglion cells (RGCs) and their axons, which leads to axon loss and visual field alterations. To date, many studies have shown the role of various elements, mainly metals, in maintaining the balance of prooxidative and antioxidative processes, regulation of fluid and ion flow through cell membranes of the ocular tissues. Based on the earlier and current research results, their relationship with the development and progression of glaucoma seems obvious and is increasingly appreciated.
- glaucoma
- neurodegenerative disease
- iron
- copper
- zinc
- selenium
- calcium
- magnesium
- molybdenum
- sodium
- potassium
- manganese
1. Introduction
Glaucoma is a heterogeneous group of chronic neurodegenerative disorders characterized by a relatively selective, progressive damage to the retinal ganglion cells (RGCs) and their axons, which leads to axon loss and visual field alterations [1,2,3,4,5,6]. It is the most common cause of irreversible blindness worldwide, affecting almost 80 million people or more than 1% of the global population [7,8,9]. It is estimated that by 2010, 1 out of 15 blind people was blind due to glaucoma, and 1 out of 45 visually impaired people was visually impaired due to glaucoma, highlighting the increasing global burden of glaucoma [4,10]. The management of glaucoma focuses on lowering IOP, the main glaucoma risk factor [5,7,11,12].
Indeed, the effectiveness of IOP-lowering therapies is questioned as they only slow down the optic nerve degeneration without significantly reversing or stopping the disease [5,7]. Thus, not only elevated IOPs play a role in the pathogenesis of glaucomatous optic nerve damage [13], which is more complex than that, and includes the contribution of many other risk factors, such as age, race, family history, environmental factors, diabetes, and myopia [1]. Moreover, many studies have shown that glaucoma is an age-related optic neuropathy showing similarities with other age-related neurodegenerations such as Alzheimer’s and Parkinson’s diseases [14]. Therefore, recent studies have also investigated the causative roles of other processes, including glutamate toxicity and glial overactivation [6,15,16,17]. Vascular dysregulation in ocular blood flow and oxidative stress have also been suggested as significant risk factors for RGCs loss in glaucoma. Moreover, mitochondrial dysfunction is another widely studied causal process in the development of glaucoma and has also been investigated as a potential drug target [9,18,19].
Another potential mechanism in glaucoma pathogenesis is alteration in the levels of various elements, mainly metals, in maintaining the balance of prooxidative and antioxidative processes, regulation of fluid and ion flow through cellular membranes of the ocular tissues [20]. Essential trace elements such as zinc, copper, selenium, manganese, chromium, cobalt, and molybdenum function as cofactors or are located in prosthetic groups of many enzymes. Metal ions such as zinc, iron, manganese, and copper are critical cofactors needed for neurotransmitter synthesis; calcium is essential for neurotransmitter release and neuroplasticity; zinc and magnesium modulate synaptic activity [16,17,18,19].
In this article, we aimed to summarize the current evidence on the role of trace elements in the pathogenesis and prevention of glaucomatous diseases. Special attention is paid to the genetic background associated with glaucoma-related abnormalities of physiological processes that regulate or involve the trace elements.
2. Trace Elements in Glaucoma
Numerous studies have reported altered serum and aqueous humor levels of trace elements in glaucoma patients (Table 1).
Table 1. Main studies assessing the role of trace elements in neurodegenerative eye diseases.
| Elements | Authors/Year | Main Results |
|---|---|---|
| Iron and copper | Farkas R.H., et al., 2014 [21] | Comparison of glaucomatous with control monkey retinas demonstrated increased mRNA expression of iron-regulating proteins. The increased levels of iron-regulating proteins in glaucoma are beneficial, because of their ability to limit iron-related oxidation. |
| Gye H.J. et al., 2016 [22] | Serum ferritin has become the preferred marker for assessing iron-related oxidative stress. High serum ferritin levels were independently associated with greater risk for glaucoma. | |
| Hara H. et al., 1999 [23] | Lomerizine increased cerebral blood flow in animal models, with no significant adverse effects. This suggests that this drug may be clinically useful in conditions associated with circulatory disturbances (such as migraine, normal-tension glaucoma, vertigo and stroke). | |
| Calcium | Al-Dabbagh N. et al., 2017 [24] | Mutation of the SPARC-related modular calcium-binding protein 2 (SMOC2) gene may be a risk factor for glaucoma, probably secondary to modification of the biological function of the Ca receptor proteins in ocular tissues. |
| Hartikainen H. et al., 2005 [25] | Calpain, a ubiquitous calcium-sensitive protease, is known to play a role in the neurodegenerative diseases such as cerebral ischemia, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, and others | |
| Head K.A. et al., 2001 [26] | Altered calcium homeostasis makes neurons more vulnerable to oxidative stress | |
| Hohberger B. et al., 2018 [20] | Plasma membrane calcium channel inhibitors were found to arrest acute axonal degeneration and improve regeneration after damage to the optic nerve | |
| Houde M. et al., 2016 [27] | In vivo studies showed the neuroprotective effects of calcium channel blockers. Moreover, beta-adrenoceptors show calcium channel blocking activity, which may be responsible for the neuroprotective effects. | |
| Hurst S. et al., 2017 [28] | Animals topically treated with calcium channel blocking agents showed a significant reduction of intraocular pressure in steroid induced glaucoma. | |
| Iqbal Z. et al., 2002 [29] | Treatment with calcium channel antagonist (nimodipine) significantly improved the visual field and color vision in glaucoma patients. | |
| Ishikawa K. et al., 2005 [30] | Oral nilvadipine increased the blood flow in distal retrobulbar arteries in normal-tension glaucoma. | |
| Hara et al., 1999, 2004 [23,31] | The Ca2+ channel blocker lomerizine increases ocular circulation and protects neuronal cells in animal models. It may be useful as a therapeutic drug against retinal diseases that involve a disturbance of the ocular circulation (such as glaucoma and retinal vascular occlusive diseases). | |
| Kitazawa Y. et al., 1989 [32] | Patients with low-tension glaucoma treated with the Ca2+ antagonist nifedipine with for 6 months. Six patients showed a constant improvement of visual field. | |
| Selenium | Phelps Brown N.A. et al., 1998 [33] | Excessive selenium supplementation may increase the glaucoma incidence |
| Prasad A.S. et al., 2014 [34] | High plasma selenium concentration and middle concentration of aqueous humour selenium was significantly associated with glaucoma. | |
| Prasanna G. et al., 2002 [35] | The mean selenium levels in aqueous humor and in serum of patients with PEX syndrome were lower than in the control group. These results may support the role of impairment in antioxidant defense system in the pathogenesis of PEX syndrome. | |
| Quigley H.A. et al., 2006 [3] | Selenium supplementation (200 mg/daily) was linked to the development of glaucoma. The risk was even higher in those who continued selenium supplementation after the trial. | |
| Zinc | DeToma A.S. et al., 2014 [36] | Retinas of pre-glaucomatous mice had greater Mg, Ca, and Zn concentrations than those of glaucomatous and greater Mg and Ca than controls |
| Grahn B.H. et al., 2001 [37] | Zn supplementation seems beneficial for the patients with diabetes. Zn as an antioxidant attenuates ROS effect, therefore it might protect retina from ROS damage, thereby being protective against DR | |
| Newsome D.A. et al., 1995 [38] | The concentrations of zinc are reduced in human eyes with signs of age-related macular degeneration (AMD) suggesting that zinc deficiency may lead to oxidative stress and retinal damage | |
| Noske W. et al., 1997 [39] | Zinc is essential in the in the eye functioning. In retina and retinal pigment epithelium zinc interacts with taurine and vitamin A and modify photoreceptor plasma membranes, regulate the light-rhodopsin reaction, modulate synaptic transmission and serve as an antioxidant. | |
| Osborne N.N. et al., 2016 [40] | Zinc could be involved in light induced retinal injury; however, the mechanisms of retinal light damage in the pathology of glaucoma remain unknown. | |
| Magnesium | Ekici F. et al., 2014 [13] | Mg may protect retinal ganglion cells from oxidative injury by combined effects on voltage-dependent calcium channels, glutathione synthesis, lipid peroxidation, and maintaining the regulation of many metabolic enzymatic reactions |
| Kumar A.R. et al., 2002 [41] | Mg is important for maintaining the structural and functional integrity of several vital ocular tissues | |
| Laganovska G. et al., 2003 [42] | Mg deficiency has been shown to cause defective neurotransmitter transport mechanism, mitochondrial dysfunction, defective Golgi body function and protein processing dysfunction, neuronal degeneration and apoptosis | |
| Lee S.H. et al., 2016 [43] | Mg plays a crucial role in Na+ and K+ transport in cells. It is an important cofactor of Na+/K+-ATPase | |
| Lenartowicz M. et al., 2015 [44] | Mg increases blood supply to the optic nerve by dilating the optic blood vessels | |
| Li X. et al., 2011 [45] | Administration of Mg twice a day for 1 month had beneficial effects on visual field | |
| Lillico A. et al., 2002 [46] | Treatment with 300 mg of Mg citrate for 1 month did not change the ocular blood flow but caused some improvement in visual field | |
| Molybdenum | DeToma A.S. et al., 2014 [36] | Increased and decreased concentrations of Molybdenum have been observed to affect the illness |
| Sodium ions/Potassium cations | Jünemann A.G.M. et al., 2018 [47] | Studies on the isolated optic nerve showed that Sodium reduced the influx of sodium and would-be effective neuroprotectants |
| Hains B.C. et al., 2005 [48] | Phenytoin (a sodium channel blocker) resulted in neuroprotection of RGCs and optic nerve axons in an experimental animal model of glaucoma | |
| Ramdas W.D. et al., 2018 [49] | The early studies showed the differences in plasma concentration between normal subjects and glaucoma patients | |
| Ribas V.T. et al., 2015 [50] | The death of RGCs is a major cause of eye neuropathies. Potassium (K+) channels play key roles in modulating the electrical properties of RG cells | |
| Roy Chowdhury U. et al., 2015 [51] | The presence of Na/K/Cl co-transport activity in trabecular meshwork (TM) cells is able to test the hypothesis that modulation of Na/K co-transport alters intracellular volume and, consequently, permeability of the TM cell membranes. | |
| Roy Chowdhury U. et al., 2016 [52] | Sodium channel blockade with phenytoin would result in neuroprotection of RGCs | |
| Roy Chowdhury U. et al., 2017 [53] | Orally delivered phenytoin was effective in protecting neurons, NS-7(4-(4-fluorophenyl)-2-methyl-6-(5-piperidinopentyloxy) pyrimidine hydrochloride a novel Na+/Ca2+ channel blocker, can protect the rat retina | |
| Roy Chowdhury U. et al., 2019 [54] | The role of intracellular Na+ overload in ischemic injury of acutely isolated rat optic nerves by evaluating electrically potentials (CAPs) from the optic nerves | |
| Saito S. et al., 2005 [55] | Cromakalim is a hypotensive agent acting via activation of Kir6.2 containing KATP channels and its effect is additive in combination with the commonly used anti-glaucoma drug latanoprost | |
| Sakamoto K. et al., 2004 [56] | Derivatives of the KATP channel were evaluated to control intraocular pressure lowering eye capabilities | |
| Salvatore S. et al., 2010 [57] | A new class of glaucoma therapeutics, opening the KATP channels, may have an effect on the trabecular meshwork and intraocular pressure regulation | |
| Sample P.A. et al., 1986 [58] | KATP channel openers—diazoxide and nicorandill—lower intraocular pressure by specifically activating the Erk1/2 pathway in ocular cells | |
| Savigni D.L. et al., 2013 [59] | Application of digoxin, a selective Na+/K+-ATPase inhibitor, for the α2β3 isoform of the enzyme, efficiently reduces the pharmacologically induced and basal intraocular pressure in rabbits | |
| Schwalfenberg G.K. et al., 2017 [60] | ATP-dependent potassium channels (KATP channels) in the mitochondrial or plasma membranes may provide protection against retinal ischemia | |
| Sheck L., Davies J. et al., 2010 [61] | ATP-dependent potassium channels (KATP channels) in the mitochondrial or plasma membranes may provide protection against retinal ischemia | |
| Shoshani Y.Z. et al., 2012 [62] | In Müller glial cells, KATP channels regulate retinal current and play a key role in retinal protection against ischemic conditions, e.g., ischemic insult | |
| Siegner S.W. et al., 2000 [63] | MaxiK channels and KATP channels were found in the eye trabecular meshwork cells | |
| Silverstone B. et al., 1981 [64] | Opening of potassium channels may play a protective role by increasing the uveal outflow | |
| Silverstone B.Z. et al., 1990 [65] | KR-31378 ((2S,3S,4R)-N’’-cyano-N(6-amino-3,4dihydro-3-hydroxy-2-methyl-2-dimethoxymethyl-2Hbenzopyran-4-yl)-N’-benzyl-guanidine) as a potent KATP-channel opener, on reducing intraocular pressure and its protective effect on RGCs | |
| Skatchkov S.N. et al., 2002 [66] | Cromakalim showed a reduction in pressure (by 30–40%) and outflow facility (by 50–80%) | |
| Sourkes T.L. et al., 1972 [16] | Effects of dexamethasone treatment on Na/K/Cl co-transport activity and co-transporter protein expression in trabecular meshwork (TM) cells | |
| DeToma A.S. et al., 2014 [36] | Mn concentration was significantly increased in patients with pseudoexfoliation (PEX) syndrome | |
| Manganese | Südhof T.C. et al., 2012 [18] | Mn level was negatively associated with glaucoma diagnosis in a population-based study of South Korean 2680 individuals |
| Demirci F.Y. et al., 2006 [67] | Study on the association between levels of three heavy metals and the occurrence of open-angle glaucoma (OAG) with low and high intraocular pressure | |
| Heavy metals [mercury (Hg), lead (Pb), cadmium (Cd), chromium (Cr), nickel (Ni), bismuth (Bi), semi-metals] | Südhof T.C. et al., 2012 [18] | Blood heavy metals level were negatively associated with glaucoma diagnosis in a population-based study on South Korean 2680 individuals |
| Tham Y.C. et al., 2014 [8] | The accumulation of Hm may be an unrecognized risk factor of non-pressure-dependent glaucomatous optic neuropathy | |
| Tykocki N.R. et al., 2010 [68] | Lead is known to cause tissue damage by oxidative stress, lipid peroxidation, and DNA damage |
Both increased and decreased concentrations had been observed by Lee et al. [43], Ceylan et al. [69], and Akyol et al. [70]. Ceylan et al. aimed to determine whether trace element levels have a role in the development of pseudoexfoliation (PEX) syndrome and pseudoexfoliation glaucoma (PEG) [69]. Levels of zinc, copper, selenium, manganese, chromium, cobalt, molybdenum, nickel, vanadium, arsenic, aluminum, mercury, cadmium, and strontium were determined in serum samples of PEX and PEG patients and control subjects using inductively coupled plasma–mass spectrometry. Mn, Mo, and Hg concentrations were found to be significantly elevated in patients with PEX. According to the authors, this may suggest the role of manganese and mercury as the “strongest determinants of PEX and molybdenum as the strongest determinant of PEG”. However, it should be noted that it is not clear what the term “determinants” would mean. No precise role of these elements in the mechanism of glaucoma formation has yet been proven. Also, Hohberger et al. analyzed concentrations of trace elements in aqueous humor samples of patients with primary open-angle glaucoma (POAG) and PEG [20]. The levels of cadmium, iron, manganese, cobalt, copper and zinc were measured by Plasma-Mass-Spectrometry. Patients with POAG and PEG had significantly higher aqueous humor levels of zinc compared to healthy controls. Iron level was significantly reduced in PEG and a significant difference between POAG and PEG was observed. According to the authors, the differences detected support the hypothesis that these elements are involved in the pathogenesis of open-angle glaucoma. No significant differences were observed in aqueous humor levels of manganese, cobalt, copper and cadmium between glaucoma patients and controls.
Vinetskaia and Iomdina used the method of spectral analysis to measure the content and composition of micro- and macro-elements in samples of lacrimal fluid from adults with open-angle glaucoma and diabetic retinopathy [71]. Changed iron, magnesium, and aluminum levels were detected and, to a lesser extent, zinc and copper in patients with diabetic retinopathy. In contrast, open-angle glaucoma was associated with reduced zinc and increased iron content.
The studies on a pigmentary dispersion animal model of PEG (DBA/2J mice) provided the basis for comparison between healthy and glaucomatous subjects in humans (Figure 1).
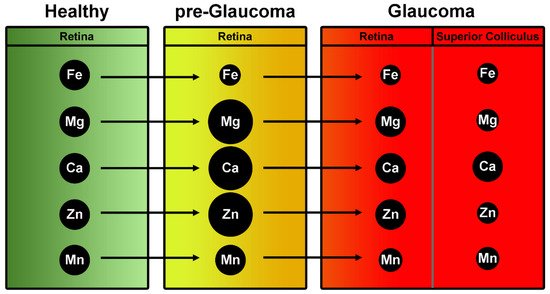
Figure 1. Summary of trace element levels on retinas of a mouse model of glaucoma (DBA2J). Green column indicates the concentration of trace elements expressed in control retinas. In yellow (middle column), levels of trace elements in retinas at pre-glaucomatous stage. In red, trace elements involvement at full-blown stage of pathology. Dimensional change of black spheres simulates the expression of elements at progressive stages of glaucoma.
DeToma et al. measured the concentrations of iron, copper, zinc, magnesium, manganese, and calcium in the retina and in the retinal projection of pre-glaucomatous and glaucomatous DBA/2J mice and age-matched controls using inductively coupled plasma mass spectrometry (ICPMS) [36]. Glaucomatous mice had lower retinal Fe concentration than pre-glaucomatous and control mice. Retinas of pre-glaucomatous mice had greater Mg, Ca, and Zn concentrations than those of glaucomatous and greater Mg and Ca than controls. Retinal Mn levels were significantly lower in glaucomatous mice compared to controls and pre-glaucomatous mice. The superior colliculus (SC, the primary retinal target in rodents and one of the earliest sites of pathology in the DBA/2J mouse) of the control mice contained more Fe, Mg, Mn, and Zn than that SC of glaucoma model DBA/2J mice.
2.1. Iron
Iron (Fe) is an essential element found in the prosthetic groups of many important metalloproteins: hemoglobin, myoglobin, and the active centers of numerous enzymes such as catalase, peroxidases, and cytochromes. Iron acts as a target for both nitrogen monoxide and carbon monoxide, two messenger molecules with crucial roles in a variety of conditions, including glaucoma [72]. Iron also participates in processes related to neurodegeneration and neuroprotection [45,73].
Iron is a redox-active agent. On the contrary, copper and zinc support the reduction of free radicals via superoxide dismutase [44,74]. The excessive consumption of iron may increase the risk of glaucoma. Thaler et al. showed that rats receiving iron before a partial optic nerve crush had significantly greater damage of the RGCs than the control group [75]. On the other hand, the iron level was significantly reduced in PEXG patients and a difference was seen when comparing POAG and PEXG individuals. The possible mechanisms of iron’s effect in glaucoma may include the activity of enzymes and other proteins involved in iron metabolism. Lin et al. (2014) investigated the association between serum ferritin level and the likelihood of a glaucoma diagnosis [76]. Their study showed that a higher serum ferritin level was associated with a greater frequency of glaucoma in a representative sample of South Koreans. The participants in this population-based study with higher serum ferritin levels (greater than 61 ng/mL) had significantly higher incidence of glaucoma than those with lower levels (less than 31 ng/mL). As the authors claimed, this finding may “help to elucidate the pathogenesis and lead to novel therapeutic approaches for glaucomatous disease”. Gye et al. investigated the association between serum ferritin levels and glaucoma in a South Korean population [22]. The studies also considered other factors (age, white blood cell count, C-reactive protein, and total vitamin D), including those related to iron metabolism (serum iron, total iron-binding capacity, transferrin saturation). It was shown that males with high serum ferritin had a higher risk for glaucoma than those with low ferritin; no difference was observed for women. Other markers of iron metabolism, such as iron level, transferrin saturation, and inflammation measures, were not associated with glaucoma. They concluded that a high serum ferritin level was associated with a high risk of glaucoma. This effect might have been related to oxidative stress and inflammation, which play a role in glaucoma development.
2.2. Copper
Copper (Cu) is generally considered to have antioxidant roles in human metabolism, as this metal is involved in eliminating superoxide radicals by the superoxide dismutase (SOD) enzyme. Cu is built-in in the active center of such enzymes as: cytochrome c oxidase; β-dopamine hydroxylase; superoxide dismutase (SOD1, SOD3); ceruloplasmin and hephaestin; tyrosinase; and lysyl oxidase [44,77]. Copper takes part in Fenton’s reaction, which forms the highly reactive hydroxyl radical. This reaction is well known for involving reduced iron as the major element that catalyzes the break-down of hydrogen peroxide resulting in the active production of free radicals [78]. Proteins that are involved in cellular copper metabolism include, among others, membrane transporters CTR1 and CTR2, metallo-chaperons, and metallothionein.
Normal copper metabolism is essential to ocular tissue health and changes in pigmentary retinopathies and high myopia [64]. Silverstone et al. measured the blood concentrations of zinc and copper and the urine concentration of copper in different groups of highly myopic patients, and in highly myopic patients with retinal detachment, showing high levels of zinc and low levels of copper in serum [65]. In the high myopic group with retinal detachment, serum zinc and copper concentrations were significantly elevated. The possible role of Cu in the development of PEX was investigated by Yildirim et al. [79], who measured the concentration of zinc and copper in lenses removed from the cataract patients. However, in contrast to Zn, the concentration in lenses from patients with PSX was significantly lower than that in patients without PSX; the mean concentration of copper did not differ significantly. Serum and aqueous humor zinc and copper concentrations of patients with glaucoma and cataract were determined by Akyol et al. [70]. The highest mean copper concentration was found in the glaucoma group. In addition, there was a significant negative correlation between the aqueous humor levels of zinc and copper in patients with glaucoma. It was concluded that an increased copper concentration and low zinc concentration might be of importance in patients with glaucoma. Since copper deficiencies have been linked with photoreceptor loss and myopia with increased scleral wall elasticity, and differences have been previously shown in Fe content in glaucomatous and healthy patients, DeToma et al. measured the levels of iron (Fe) and copper (Cu) in ocular structures of healthy [36], pre-glaucomatous and glaucomatous DBA/2J mice. No differences in retinal Cu levels were shown between any of the groups in the study but the glaucomatous mice had lower retinal Fe concentrations than pre-glaucomatous and control mice.
The concentration of copper and iron was also measured in the aqueous humor of rabbit eyes treated with steroids; the Cu and Fe levels in relation to a steroid-induced increase in intraocular pressure (IOP) were evaluated [29]. After 30 days of steroid treatment, the mean concentration of copper in the aqueous humor of steroid-treated rabbits was significantly lower compared to the control group. However, the concentration of iron was not different. As concluded, lowering copper concentration resulting from steroid treatment may play an important role in the maintenance of high IOP in rabbits’ eyes, which may explain the role of Cu in the pathogenesis of OAG.
2.3. Calcium
Calcium participates in an intracellular signaling system by acting as a diffusible second messenger. Calcium and calcium signaling play a crucial role in the nervous system [80]. In neurons, intercellular calcium signals—the transient changes in cytoplasmic free calcium—propagates the calcium signal to neighboring cells. Moreover, activation of calpain, a ubiquitous calcium-sensitive protease, is known to play a role in neurodegenerative diseases such as cerebral ischemia, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, and others [81]. There is now evidence that the pathological progression of neurodegeneration in glaucoma also includes the Ca2+-dependent mechanisms [14]. In contrast to some other macro- or micronutrients indispensable to life, the mechanism of calcium’s effect on the incidence of glaucoma and its course has been intensively studied [82]. The relationship between glaucoma occurrence and excessive consumption of calcium has been reported; on the other hand, calcium supplementation led to a reduction in human susceptibility to glaucoma [5,83].
Crish and Calkins reviewed the results of studies showing that the pathological neurodegeneration in glaucoma might result from Ca2+ dependent mechanisms [14]. Calcium homeostasis and calcium signaling are related closely to neuronal functions such as synaptic transmission or cell survival. It was shown that disturbed calcium homeostasis might make neurons more vulnerable to oxidative stress [84]. It is supposed that the calcium channel-blocking agents (e.g., nimodipine and brovincamine) or nilvadipine (an L-type Ca2+ blocking agent) may retard the age-related disturbance of calcium homeostasis and thus decrease the incidence of open-angle glaucoma, which is a well-known age-dependent disease.
Plasma membrane calcium channel inhibitors were found to arrest acute axonal degeneration and improve regeneration after damage to the optic nerve [50,59]. Numerous experiments have shown that the application of calcium channel blockers (CCBs, e.g., verapamil) caused significant reduction of intraocular pressure. These generally cause widening of blood vessels in the eye and increase ocular blood flow in experimental animals and patients with open-angle glaucoma. CCBs, such as nimodipine, brovincamine, and nilvadipine, had beneficial effects on visual function not only in normal humans but also in patients suffering from glaucoma. In vitro studies showed that calcium channel blockers exerted protective effects on neurons undergoing apoptosis and necrosis. In experimental animal models also, neuroprotective effects were shown. In these experiments, beta-adrenoceptor antagonists (e.g., betaxolol) show calcium channel blocking activity, which may be responsible for the neuroprotective effects (Araie and Mayama) [85]. The results obtained by Ganekal on rabbits topically treated with verapamil [86] and diltiazem showed that these calcium channel blocking agents significantly reduced intraocular pressure in steroid-induced glaucoma.
The role of mitochondrial dysfunction has been studied in the development of glaucoma. Many results indicated that reactive calcium signaling and the generation of free radicals and other active oxygen species regulated by the mitochondria [28,81] are critical in glaucoma pathogenesis [9]. The authors suggest that extensive studies of glaucoma and its relationship with aging, mitochondrial diseases, and calcium signaling dysfunction should lead to the development of new drugs based on calcium channel regulators used to treat glaucoma and other neurodegenerative diseases of the retina. They suggest that the utilization of mitochondrial-specific calcium channel regulators may help treat glaucoma and other neurodegenerative eye diseases.
Calcium entry blockers are probably the most frequently and most accurately tested substances used as anti-glaucoma medications. Ca2+ entry blockers are potent drugs dilating ocular blood vessels, which have been demonstrated both in in vivo and in vitro studies [85,87]. This process is called vasodilation relaxation of the smooth muscles in ocular vessels and depends mainly on the extracellular Ca2+ flow. Ca2+ entry blockers may play a role in relaxing the retinal, long posterior ciliary arteries to improve the ocular circulation in vascular diseases manifested by increased tension of the eye blood vessels. Treatment with nimodipine, a centrally active calcium channel antagonist, significantly improved the visual field and color vision in glaucoma patients [88]. However, this drug was not shown to alter the blood flow dynamics in macular vessels. Yamamoto et al. investigated the effect of nilvadipine [89], a calcium-channel blocker, on the hemodynamics of retrobulbar vessels in normal-tension glaucoma. Oral nilvadipine increased the blood flow in distal retrobulbar arteries in normal-tension glaucoma without affecting more proximal blood vessels. The conclusion was that nilvadipine might have a beneficial effect on the blood flow in retrobulbar vessels in normal-tension glaucoma.
In the experiments carried out both in vitro and in vivo on animal models, Hara et al. reported that lomerizine [23,31], a Ca2+ channel blocker, increased ocular circulation and protected neuronal cells. In rabbits with disturbed circulation, lomerizine and other Ca2+ channel blockers increased the ocular circulation and protected the optic nerves. Moreover, lomerizine reduced damage of the retina in rats, presumably through a Ca2+ channel blocking effect, which may involve an improvement in the ocular circulation. The authors claim that lomerizine may be useful as a therapeutic drug against ischemic retinal diseases (such as glaucoma and retinal vascular occlusive diseases) that involve a disturbance of the ocular circulation. Kitazawa et al. evaluated the effects of nifedipine, a Ca2+ antagonist, on the visual field in low-tension glaucoma [32]. Patients received 30 mg/day per os for 6 months. Six patients showed a constant improvement in the visual field. Of the substances known to benefit the survival of damaged neurons used by Osborne et al. to treat glaucoma [90], beta-blockers were shown to reduce the sodium and calcium influx process. Betaxolol was shown to be the most effective antiglaucoma medicine, reducing sodium and calcium influx. Topical application to rat eyes of levobetaxolol and timolol, beta-adrenergic receptor inhibitors (beta-blockers), both known to influence sodium and calcium flux in cell membranes, was shown to alleviate the effects of ischemia-reperfusion injury. Based on these results, the authors concluded that these drugs can attenuate ganglion cell death in glaucoma. They also suggest that betaxolol and, to a lesser extent, timolol may be effective neuroprotectants and that the mechanism of their action is by blocking the calcium and sodium influx into neural cells. Studies carried out by Osborne et al. on the isolated optic nerve also showed that beta-blockers which reduce the influx of sodium are particularly effective neuroprotectants [90]. They also reduce the influx of calcium which additionally benefits the survival of insulted neurons. Of the used antiglaucoma drugs, betaxolol was shown to be the most effective substance at reducing sodium/calcium influx.
Siegner et al. determined the effect of different calcium channel blockers (representative members from six classes) on intraocular pressure in the eyes of the macaque (Macaca fascicularis) [63]. Moreover, the effect of other antiglaucoma medications was studied in combination with verapamil to determine their effect on intraocular pressure. This study showed that all classes of calcium channel blockers significantly lowered intraocular pressure. The conclusion was that calcium channel blockers and combinations of antiglaucoma medications with verapamil might provide a useful medication for reducing intraocular pressure in patients with ocular hypertension or POAG. In monkeys also, but with laser-induced unilateral glaucoma, flunarizine, a nonselective calcium channel blocker, was shown to reduce intraocular pressure in a dose-dependent manner when administered to glaucomatous eyes [91]. However, in this case, a hypotensive effect on the normal, untreated eyes was also observed.
Different experimental approaches have been used to investigate the mechanism of the interaction of drugs used to treat glaucoma on the functioning of calcium channels in nerve cells. Melena et al. investigated the affinity of several antiglaucoma drugs, including betaxolol, timolol, and others, for voltage-dependent Ca2+ channels (VDCCs) using radioligand binding assays [92]. Of the antiglaucoma drugs investigated, betaxolol displayed the greatest L-type VDCC-blocking activity, and this may account for some of its actions in ocular hemodynamics. Hong et al. investigated various commercial antiglaucoma drugs and their effects in inhibiting glutamate-induced intracellular free Ca2+ increase in cultured N1E-115 neuroblastoma cells [93]. They found that betaxolol, dipivefrin, brimonidine and timolol have remarkable effects on inhibiting the glutamate-induced Ca2+ increase, and also decreased the basal calcium concentration in the neuroblastoma cells in vitro. These results indicate that betaxolol, dipivefrin, and brimonidine may have neuroprotective effects in inhibiting the glutamate-induced cell damage resulting from the excessive Ca2+ influx.
The effect of calcium channel blockers on the visual field, optic discs, and intraocular pressure was analyzed by Liu et al. [94]. There was no apparent difference in intraocular pressure, visual field, or optic discs when comparing patients using calcium channel blockers with the control group. Thus, this study showed no beneficial effect of calcium channel blockers on the course of the disease in patients suffering from glaucoma. The results of most of these studies showed that calcium channel blockers are effective in glaucoma treatment. They have shown a positive effect on ocular blood flow and visual field in normotensive glaucoma (NTG) [13,95]. However, some unwanted side effects such as hypotension and bradycardia restricted the use of calcium channel blockers for the treatment of glaucoma [13,96].
2.4. Magnesium
Magnesium plays a significant role as a cofactor for more than 350 enzymes [97]. The enzymes involved in ATP production and hydrolysis are also magnesium-dependent. Magnesium deficiency also results in increased oxidative stress and inducible NOS stimulation that can further contribute to the initiation and progression of ocular pathologies such as cataract, glaucoma, and diabetic retinopathy. Moreover, magnesium deficiency may be the cause of vasoconstriction. This can lead to tissue ischemia and tissue death, which is one of the pathogenic factors in POAG. Furthermore, magnesium deficiency is a causative factor in increasing oxidative stress and inducing nitric oxide synthase (NOS) activity that can further contribute to the initiation and progression of ocular pathologies such as cataract, glaucoma, and diabetic retinopathy. Reduced Na+/K+-ATPase activity associated with magnesium deficiency has been shown to cause defective neurotransmitter transport mechanism, mitochondrial dysfunction, defective Golgi body function, protein processing dysfunction, neuronal degeneration, and apoptosis [41]. Moreover, the loss of Na+/K+-ATPase, resulting from Mg deficiency, causes intracellular Na+ accumulation and release of mitochondrial Ca2+ ions, which results in increased cytoplasmic Na+ and Ca2+ [13,98]. Increased intracellular Ca2+ and Na+ further cause cellular swelling leading to RGCs apoptosis.
The improvement of ocular blood flow and prevention of ganglion cell loss make magnesium a good candidate for glaucoma treatment [13]. Mg improves blood flow by modifying endothelial function via endothelin-1 (ET-1) and endothelial nitric oxide (NO) pathways. Mg also exhibits a neuroprotective role by blocking N-methyl-D-aspartate (NMDA) receptor-related calcium influx and inhibiting glutamate release. It protects the cell against oxidative stress and apoptosis.
Mg is important for maintaining the structural and functional integrity of several vital ocular tissues [13,97]. The magnesium content in the lens, especially in its peripheral part, is higher than that in the aqueous and vitreous humor of the eye. Magnesium has also been shown to play a critically important role in retinal functions. Reduction in blood flow was observed in ocular tissues—retina, optic nerve, iris, and choroid—in various ocular disorders, including glaucoma. Mg was shown to enhance ocular vasodilation and optimize ocular blood flow. Therefore, Mg supplementation may have a therapeutic value in glaucoma and may protect the ocular tissues by regulating ocular blood flow and reducing oxidative stress. This is supported by the finding that magnesium facilitates blood flow by reducing cytokine levels and free radical production and preventing intracellular calcium entry [99].
Head reported that glaucoma patients for whom the optic nerve damage is caused by the decreased blood supply to the optic nerve could benefit from supplemental magnesium, which is a “physiological calcium blocker”. Gaspar et al. evaluated the effect of magnesium in POAG patients and those with normal-tension glaucoma [100]. Administration of magnesium twice a day for one month had beneficial effects on the visual field. Moreover, all parameters studied, blood cell velocity, cold-induced blood flow cessation, and the number of capillaries per microscopic field and digital temperature, improved significantly. The authors concluded that magnesium improves the peripheral circulation, widens the visual field, and has a relieving effect on glaucoma patients.
Summing up, the above data speaks for the beneficial effect of magnesium in relieving glaucoma symptoms. Mg may protect RGCs from oxidative injury by combined effects on voltage-dependent calcium channels, glutathione synthesis, lipid peroxidation, and by maintaining the regulation of many metabolic enzymatic reactions. Both improvements in ocular blood flow and prevention of ganglion cell loss would make magnesium a good candidate for glaucoma treatment. However, as most authors emphasize, further studies are needed on the possible beneficial effect of Mg in curing glaucoma. What is more, some disappointing results have been obtained. In the studies involving normal-tension glaucoma (NTG) patients, Aydin et al. have shown that treatment with 300 mg of magnesium citrate for one month did not change the ocular blood flow (although it caused some improvement in the visual field) [101]. These results showed that oral magnesium therapy might improve the visual field in normotensive glaucoma, but it does not seem to affect ocular blood flow in patients with NTG. Another mechanism could therefore contribute to this effect.
2.5. Molybdenum
The concentrations of molybdenum (Mo) were significantly increased in the blood of patients with PEX [69]. Based on this result, the authors postulated molybdenum as a strong determinant of PEX. Molybdenum is part of some of the metallo-flavoproteins—xanthine oxidase, involved in purine metabolism, and (besides iron) aldehyde dehydrogenase, catalyzing the oxidation of acetaldehyde to acetic acid. However, so far, no precise role of Mo in glaucoma pathophysiology has been proven.
2.6. Zinc
Zinc (Zn) is an essential microelement present in the active centers of about 200 enzymes, involved in many signaling pathways [102]. Zinc is generally considered an antioxidant factor involved in the process of elimination of superoxide radicals [103]. Zinc is a trace element that appears to play an integral role in maintaining normal ocular function [37]. In the eye, zinc is essential in the retina, choroid, cornea, and lens [104]. In the retina and retinal pigment epithelium, zinc interacts with taurine and vitamin A and modifies photoreceptor plasma membranes, regulates the light-rhodopsin reaction, modulates synaptic transmission, and serves as an antioxidant. Because of its elevated levels in POAG and PEG, Zn may be related to glaucoma pathogenesis. Matrix metalloproteinases are zinc-containing enzymes capable of degrading all kinds of extracellular matrix proteins and processing several bioactive protein molecules, some of which play a decisive role in glaucoma pathogenesis.
Newsome et al. and Prasad demonstrated that zinc concentrations are reduced in human eyes with signs of age-related macular degeneration (AMD) and suggested that zinc deficiency may lead to oxidative stress and retinal damage [34,38]. Studies of dietary supplementation of zinc gave ambiguous results, but it seems that it can help alleviate the effects of AMD in the elderly.
As reviewed by Brown et al. [33], zinc has a role in retinal metabolism and may be beneficial in macular degeneration. Research cited in this review showed that altered serum levels of some trace elements, e.g., copper and zinc, may impact the incidence and progression of glaucoma disease. Both increased and decreased concentrations have been observed to affect the illness [47,73,74].
Zinc seems to play a role in glaucoma pathophysiology [105]. In some forms of glaucoma, the cones of human eyes were shown to contain high amounts of zinc, and the damage to blue cones might cause the release of this metal to the extracellular space [58]. Zinc could also be involved in light-induced retinal injury; however, the mechanisms of retinal light damage in the pathology of glaucoma remain unknown [106]. Neurons overloaded with zinc probably exist in the mammalian retina; however, it appears unlikely that the death of RGCs after ischemia, which occurs in glaucoma, is due to an influx of zinc originating from zinc-enriched neurons. The mean concentration of zinc in the lens from patients with PEX was significantly lower than that in the lenses of patients without PEX. The decreased content of zinc could increase oxidative stress that is thought to contribute to the development of PEX in cataract patients [79].
2.7. Selenium
Selenium (Se) is an essential mineral incorporated into at least 25 distinct proteins, mainly as selenocysteine residue. These proteins include glutathione peroxidases (GPx), iodothyronine deiodinases, and thioredoxin reductases. Se is best known for its crucial role in the GPx enzyme system, the major antioxidant defense system. Selenium is also incorporated into other selenoproteins, which prevent cellular damage through their antioxidative properties [49,107].
It was found that high plasma Se is associated with glaucoma and the intake of selenium might increase the risk of glaucoma [49]. Selenium supplementation was shown to be linked to glaucoma [46,47]. Moreover, selenium concentration in aqueous humor of patients with POAG and PEG was higher than in healthy individuals. Bruhn et al. compared selenium levels in blood plasma and aqueous humor in patients with and without POAG [108]. High plasma selenium concentration was significantly associated with glaucoma. Yilmaz et al. investigated the levels of selenium in aqueous humor, conjunctiva, and serum of patients with PEX and control subjects [109]. The mean Se levels in aqueous humor of patients with PEX syndrome were significantly lower than in the control group. However, serum Se levels in PEX patients and healthy controls did not differ significantly (a tendency toward lower Se in PEX was observed). The authors conclude that the reduced levels of Se may suggest the impairment of the antioxidant defense system in the PEX.
Laganovska et al. estimated the serum kynurenine, neopterin, and selenium concentrations in aqueous humor from the anterior chamber of the eye, and selenium content in lenses from operated cataract patients with and without PEX [42]. Significantly increased kynurenine and neopterin concentrations were observed in patients with PES compared to healthy controls. They also had the lowest content of selenium in the serum and lens compared with patients without PEX. The decreased content of selenium may elicit immune system activation via increased oxidative stress, as was indicated by the increased concentration of kynurenine and neopterin.
Conley et al. determined the effects of selenium on trabecular meshwork cells, a likely site of pathology for glaucoma [110]. Human trabecular meshwork (HTM) cells were treated with selenium, in the form of methyl-seleninic acid (MSeA), at a concentration considered to be physiological. Selenium uptake by cells, alterations in protein secretion, intracellular signaling, and cell morphology were monitored. Moreover, the role of integrin signaling in MSeA-treated cells was investigated. Selenium-induced morphological changes occurred before alterations in protein secretion and intracellular signaling. Selenium was shown to influence several indicators of HTM cell homeostasis but did not affect cell viability.
The association between selenium and glaucoma seems to be complex and not well-understood [61]. In the study performed in 1996, involving 1312 patients, selenium supplementation (200 mg/daily) was linked to the development of glaucoma. The risk was even higher in those who continued selenium supplementation after the trial. Other case-control studies cited in this article (Bruhn et al.) also showed that selenium supplementation might carry a risk of glaucoma [108]. In summary, there are both biological and human studies suggesting that selenium supplementation is linked with an increased incidence of glaucoma. The association between high plasma selenium level and glaucoma confirms the suggestion that selenium supplementation may carry a risk of developing glaucoma.
2.8. Sodium and Potassium
Sodium ions (Na+) are involved in neuronal impulse transmission, affect the osmotic pressure of body fluids, and increase the hydration of cellular colloids. Potassium cations (K+) also participate in the transmission of impulses by neurons. Early studies showed the differences in plasma potassium concentration between normal subjects and glaucoma patients [111]. This could suggest a critical role of potassium ions in the pathogenesis of glaucoma. Indeed, numerous subsequent studies have confirmed the important role in glaucoma of this element, in cooperation with other ions (Na+, Mg+, and Ca2+) and their transport through cellular and mitochondrial membranes.
Potassium (K+) channels play key roles in modulating the electrical properties of RG cells [112]. A number of eye diseases, including glaucoma and ischemic optic neuropathy, may cause injury or the death of these cells, and subsequently permanent visual dysfunction. Studies have identified a number of K+ channels in RG cells, including inwardly rectifying K+ (Kir), ATP sensitive K+ (KATP), tandem pore domain K+ (TASK), voltage-gated K+ (Kv), etheràgogo (Eag), and Ca2+ activated K+ (K/Ca) channels, which are involved in the controlling of RGCs excitability, their maintenance, survival, and neuroprotection. Considering these important roles of K+ channels, it is supposed that the study of their regulation in RG cells may result in explaining some pathophysiological mechanisms and provide new means of their protection in glaucoma patients.
In early studies, O’Donnell et al. conducted experiments to evaluate the presence of Na/K/Cl co-transport activity in trabecular meshwork (TM) cells and to test the hypothesis that modulation of Na/K co-transport alters intracellular volume and, consequently, permeability of the TM cell membranes [113]. They demonstrated that bovine and human TM cells exhibit Na/K/Cl co-transport activity that is modulated by a variety of hormones and neurotransmitters. These findings suggest that Na/K/Cl co-transport of TM cells is of central importance to the regulation of intracellular volume and TM permeability. Defects of Na/K/Cl cotransport may underlie the pathophysiology of glaucoma.
Magnesium plays a crucial role in Na+ and K+ transport in cells. It is an important cofactor of Na+/K+-ATPase, responsible for the active transport of Na+ out of the cell membrane in exchange with K+ [13,114]. Mg2+ deficiency leads to functional loss of Na+/K+-ATPase and causes intracellular Na+ accumulation and release of mitochondrial Ca2+ ions, which results in increased cytoplasmic Na+ and Ca2+ [98]. Increased intracellular Ca2+ and Na+ further cause cellular swelling leading to retinal ganglion cell apoptosis.
An excessive influx of sodium inside the cells through voltage-gated sodium channels is an important event in the cascade leading to degeneration of axons. Hains and Waxman tested the hypothesis that sodium channel blockade with phenytoin would result in neuroprotection of RGCs and optic nerve axons in an experimental model of glaucoma in Wistar rats with chronic elevation of intraocular pressure (IOP), leading to optic nerve damage [48]. The study showed that orally delivered phenytoin was effective in protecting neurons. Saito et al. investigated whether NS-7 (4-(4-fluorophenyl)-2-methyl-6-(5-piperidinopentyloxy) pyrimidine hydrochloride [55], a novel Na+/Ca2+ channel blocker, can protect the rat retina. They showed that NS-7 has neuroprotective effects against retinal damage resulting from subjection to ischemia. The authors also suggested that NS-7, can be used as an agent for treating acute ischemic retinopathy, including diseases associated with high intraocular pressure, such as acute angle-closure glaucoma.
Dong and Hare characterized the role of intracellular Na+ overload in the ischemic injury of acutely isolated rat optic nerves by evaluating electrically potentials (CAPs) from the optic nerves [115], a measure of optic nerve function. When a rapidly reversible Na+ channel blocker was present during one hour of oxygen and glucose deprivation, the recovery of the potentials was significantly enhanced. The results showed that intracellular Na+ overload played a significant role in the ischemic injury of optic nerves. This may depend at least partially upon Ca2+ influx from the extracellular space.
Studies on the isolated optic nerve showed that substances that reduce the influx of sodium were effective neuroprotectants [90]; beta-blockers were shown to reduce sodium influx into cells. They also reduce the influx of calcium which additionally benefits the survival of insulted neurons. Of the used antiglaucoma drugs, betaxolol was the most effective at reducing sodium/calcium influx. Topical application of levobetaxolol and timolol to rats, which attenuated the effects of ischemia injury, also appeared highly effective. These studies showed that levobetaxolol is a more effective neuroprotectant than timolol because of its greater capacity to block sodium and calcium influx.
KATP channel openers are considered as potential therapeutics for the treatment of glaucoma. They were shown to lower intraocular pressure (IOP) in animal models and in vitro cultured anterior segments of human eyes. A study by Chowdhury et al. showed that cromakalim is a hypotensive agent acting via activation of Kir6.2 containing KATP channels [51]. Its effect is additive in combination with the commonly used anti-glaucoma drug latanoprost. In another study, Chowdhury et al. have used derivatives of the KATP channel opener cromakalim and evaluated their intraocular pressure lowering capabilities [52]. The phosphate derivatives of cromakalim proved to be effective in lowering IOP in mouse and rabbit models in vivo. No toxic effects on cell structure or the aqueous humor outflow pathway were observed after the treatment, suggesting that these drugs are strong candidates for ocular hypotensive agents. The recent study by Chowdhury et al. showed that other KATP channel openers—diazoxide and nicorandill—lower intraocular pressure by specifically activating the Erk1/2 pathway in ocular cells [53,54], the signaling axis, which is one of the most important pathways involved in survival and proliferation of various cells. The obtained results indicate a mechanism according to which KATP channel openers, targeting the Erk1/2 signal transduction pathway, can treat hypertensive ocular diseases such as glaucoma.
Katz et al. described the Na+/K+-ATPase inhibitor, digoxin, selective for the α2β3 isoform of the enzyme [116], and showed that its application efficiently reduces the pharmacologically induced and basal intraocular pressure in rabbit eyes. This work confirms that selective digoxin derivatives, which effectively inhibit the Na+/K+-ATPase and reduce aqueous humor production in the eye, may have the potential for glaucoma drug therapy. Skatchkov et al. and Yamauchi et al. showed that ATP-dependent potassium channels (KATP channels) in the mitochondrial or plasma membranes might provide protection against retinal ischemia [66,117]. In the Müller glial cells, KATP channels regulate retinal current and play a key role in retinal protection against ischemic conditions, e.g., ischemic insult [56,66,117]. In other studies, MaxiK channels and KATP channels were found in the eye trabecular meshwork cells [118,119], and the opening of these potassium channels, it was postulated, might play a protective role by increasing the uveal outflow.
Choi et al. investigated the effect of KR-31378 ((2S,3S,4R)-N’’-cyano-N(6-amino-3,4dihydro-3-hydroxy-2-methyl-2-dimethoxymethyl-2Hbenzopyran-4-yl)-N’-benzyl-guanidine) a benzopyran analog [120] and a potent KATP-channel opener, on reducing intraocular pressure and its protective effect on RGCs. They showed that under the condition of chronic ischemia, the cell density in the KR-31378–treated group was higher than that in the non-treated group, and intraocular pressure was reduced. In the acute retinal ischemia model RGCs in KR-31378–treated retina were protected from ischemic damage. These results showed that KR-31378 exerts both a neuroprotective effect and a pressure-reducing effect. The authors suggest that KR-31378 may be used to improve glaucoma therapies.
Functional disorders of KATP channels can be counted as events leading to glaucoma; these channels may also play a role in regulating intraocular pressure. KATP channel openers were shown to have both hypotensive and neuroprotective properties. Therefore, they have the potential to be a new class of glaucoma therapeutics. Roy Chowdhury et al. aimed to determine whether the opening of KATP channels affected the trabecular meshwork and intraocular pressure regulation. They treated in vitro the human anterior segment perfusates with KATP channel openers—diazoxide, nicorandil, and cromakalim. All these treatments showed a reduction in pressure (by 30–40%) and outflow facility (by 50–80%). In a similar experiment, latanoprost, the active component of the glaucoma drug Xalatan, increased the outflow facility by 67% [121]. Pressure reduction with KATP channel openers was reversible and was completely inhibited by the channel closer glibenclamide. Their results have shown that KATP channel openers have the potential to become a new class of glaucoma therapeutics that can lower intraocular pressure and protect the RGCs and the optic nerve from neuronal degeneration. Putney et al. evaluated the effects of dexamethasone treatment on Na/K/Cl co-transport activity and co-transporter protein expression in trabecular meshwork (TM) cells [122]. The authors found that the exposition of human and bovine TM cells to dexamethasone stimulates Na/K/Cl co-transport activity. Moreover, they found that two–five days of treatment with dexamethasone increased the co-transporter protein expression while longer exposures decreased the protein levels below controls’. The authors’ findings suggest that dexamethasone may be exerting its effect by altering Na/K/Cl cotransport function in TM cells, which may be an underlying factor in steroid-induced glaucoma.
2.9. Manganese
Manganese (Mn) is a redox-active metal and can promote free radical formation, leading to oxidative stress. Manganese is a component of the superoxide dismutases (MnSODs), which catalyze the dismutation reaction of superoxide anion radical to hydrogen peroxide and oxygen. Concentrations of manganese have a significant effect on the activity of antioxidant enzymes and thus on the fight against oxidative stress [123].
Ceylan et al. found that manganese concentration was significantly increased in patients with PEX [69]. The authors suggested that Mn levels had a strong association with PEX and that the increased levels of serum Mn may have a possible role in the pathobiology of PEX. When the three groups (PEX, PEG, and control) were analyzed together, Mn concentration was significantly different. On the other hand, they observed that Mn levels were almost the same in the PEG and the control groups. Moreover, concentrations of all elements measured fell into the respective reference intervals, except for the Mn level of PEX patients, which was slightly above the upper limit for Mn. On the other hand, as reported by Lin et al., blood manganese level was negatively associated with glaucoma diagnosis in a population-based study on 2680 South Korean individuals [124]. These findings suggest that a low blood manganese level could be associated with greater susceptibility to glaucoma. This element may have some, as yet undetermined, relationship with the occurrence of this disease.
2.10. Heavy Metals
In biology and medicine, the term “heavy metals” generally refers to elements used in industry and characterized by toxicity to humans or the environment. Heavy metals include metals, e.g., mercury (Hg), lead (Pb), cadmium (Cd), chromium (Cr), nickel (Ni), copper (Cu), zinc (Zn), and bismuth (Bi); semi-metals, e.g., arsenic (As), tellurium (Te); and even non-metals (selenium; Se). The toxic action of these “metals” is associated with their ability to accumulate in the body, including bones, kidneys, and brain. Their salts and oxides can cause severe poisoning and acute and chronic diseases of the circulatory system, nervous system, and kidneys.
Heavy metals have the capacity to replace previously bound metals and change the concentrations of other metals in patients’ tissues, including ocular tissues [125]. Therefore, in several publications appearing in ophthalmic literature, the influence of heavy metals on the incidence and morbidity of glaucoma was examined.
In the study by Ceylan et al., the concentration of several trace elements was measured in the blood of PEX and PEG patients [69]. From the elements considered to be heavy metals, Hg was found to be significantly increased in patients with PEX compared to controls. In this study, the levels of Zn, Cu, Se, Cr, Ni, and As were not significantly different in the PEX patients compared to healthy controls. The above-mentioned study by Lin et al. also investigated the association between blood levels of some heavy metals, mercury, cadmium, lead, and arsenic, in 2680 South Korean individuals [124]. In this study blood mercury level was positively associated with glaucoma diagnosis, thus confirming its negative impact on human health. Besides, these findings suggest that a higher blood mercury level is associated with an increased incidence of glaucoma. No definitive association was identified for blood levels of cadmium or lead, or urine arsenic level. The association between levels of three heavy metals and the occurrence of open-angle glaucoma (OAG) with low- and high intraocular pressure was studied by Lee et al. (in South Korean teenagers [43]. The mean of blood cadmium levels was significantly higher in subjects with OAG than that of the non-glaucomatous group. In contrast, there were no significant differences in blood lead and mercury. The results of this study suggest that cadmium toxicity could also play a role in glaucoma pathogenesis, particularly in male OAG patients with low baseline intraocular pressure.
Yuki et al. showed that lead accumulation might be an unrecognized risk factor of non-pressure-dependent glaucomatous optic neuropathy [126]. They showed some lead accumulation in Japanese open-angle glaucoma patients. A higher hair lead level, which reflects the total body burden, was associated with POAG in females, especially those with low-tension glaucoma. Lead is known to cause tissue damage by oxidative stress, lipid peroxidation, and DNA damage [127]. Nevertheless, the precise role of lead and other heavy and toxic metals on the appearance of glaucoma remains unknown.
Although the results of these studies indicate the association of elevated levels of some heavy metals with the occurrence of glaucoma, we do not know whether this is due to their general toxicity or whether they have a particular effect on the glaucoma pathogenesis (Figure 2).
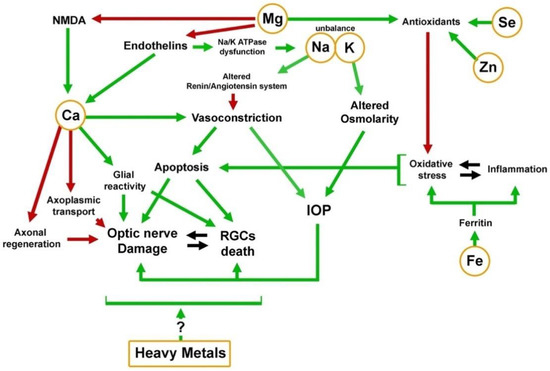
Figure 2. Mechanistic scheme of the activity of elements in the pathological background of glaucoma. Green arrows indicate involvement of the element in activational process; Red arrows indicate inhibition.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094323
