Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Medicinal
Green synthetic protocol refers to the development of processes for the sustainable production of chemicals and materials. For the synthesis of various biologically active compounds, energy-efficient and environmentally benign processes are applied, such as microwave irradiation technology, ultrasound-mediated synthesis, photo-catalysis (ultraviolet, visible and infrared irradiation), molecular sieving, grinding and milling techniques, etc.
- drug
- green chemistry
- microwave
- oxadiazole
- synthesis
- biological activities
1. Introduction
The green chemistry approach refers to the utilization of a set of principles that reduces the generation of chemical hazardous during the design, manufacture and use of chemical substances. This protocol plays a major role in controlling environmental pollution by using safer solvents, catalysts, suitable reaction conditions and thereby increases the atom economy and energy efficiency of the synthetic process. Hence, microwave-assisted synthesis followsthe green chemistry approach as it makes the synthetic process eco-friendly by reducing environmental pollution [1]. Microwave radiation energy offers significant benefits to carry out drug synthesis, including increased reaction rates, product yield enhancements, and cleaner reactions.The chemical transformations which take hours, or even days, to complete can now be completed in minutes with the help of microwave heating [2].
Similarly, the ultrasonic irradiation method is applied to accelerate various chemical reactions, including both homogeneous and heterogeneous systems. The use of ultrasound in organic synthesis involves specific activation based on the physical phenomenon, i.e., acoustic cavitations.In contrast, photo-catalytic reactions involve the use of ultra-violet, visible light and infrared radiation to generate new medicinally active compounds with diverse molecular structures. To carry out a photochemical reaction, the UV-visible spectra of the photoactive compounds are recorded. The photoactive compound is the molecule that can be electronically excited and undergoes chemical reaction from its excited state [3].
The grinding technique is also considered a green synthetic method to perform chemical reactions under solvent-free conditions with high product yield. Grinding of the recanting substances for a chemical reaction can be carried out by using mortar and pestle or by using a high-speed vibrating mill. Due to the collision between the reacting molecules, the chemical reaction is carried forward [4]. A milling technique like ball milling is considered to be one of the automated forms of mortar and pestle. In the case of theball mill, the reacting materials are placed in a reaction vessel equipped with grinding balls and covered with a lid. The vessel is allowed to shake at high-speed to carry out the chemical reactions [5].
2. Green Chemistry Approaches
There are various green chemistry approaches to carry out different chemical reactions that include microwave irradiation (MWI), ultrasonication, photo-catalysis, grinding and milling methods (Figure 1). By applying these technologies, organic reactions become more efficient and economic by enhancing the rate of reaction with reduced reaction time and high product yield. Synthetic approaches like grinding or milling techniques involve the application of mechanochemistry for the rapid, clean, efficient and solvent-free synthesis of various biologically active compounds [7].
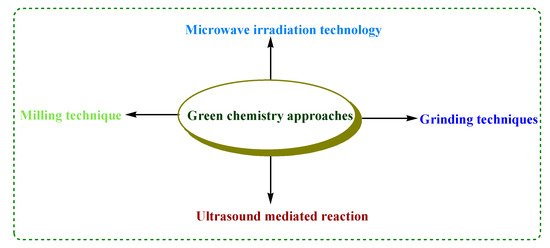
Figure 1. Green chemistry approaches.
During the year 1991, the Environmental Protection Agency (EPA) and the National Science Foundation (NSF) initiated the Green Chemistry Program. P.T. Anastas and J.C. Warner have formulated twelve major principles of green chemistry to reduce or eliminate the risk of chemical hazards and environmental pollution [8,9,10,11].
-
Prevention of waste or byproducts: It is essential to carry out the synthesis in such a way that the formation of waste or byproducts is less or absent.
-
Atom economy: It represents the design of synthetic methods to maximize the incorporation of reactants (starting materials and reagents) to get the final products.
-
Use of less hazardous and toxic chemicals: Various synthetic methods should be designed properly so that the use and generation of substances have less or no toxic effect on human health and the environment.
-
Designing of Safer chemicals: The design of the chemical product should preserve efficacy while reducing toxicity.
-
Selection of Safer solvents: Avoid the use of auxiliary materials (solvents, extractants) if possible, or otherwise, make them innocuous.
-
Energy efficiency: Energy requirements should be minimized and conduct synthesis at ambient temperature and pressure.
-
Renewable feedstock: Raw materials should be renewable.
-
Reduce derivatives: Unnecessary derivatization should be avoided where possible.
-
Smart catalysis: Selectively catalyzed processes are superior to stoichiometric processes.
-
Biodegradable design: The design of chemical products should be in such a way that these can be degradable to innocuous products when disposed of.
-
Real-time analysis for pollution prevention: Monitor the processes in real time to avoid excursions leading to the formation of hazardous substances.
-
Prevention of hazards and accidents: Materials used in a chemical process should be selected to minimize hazardsand risk for chemical accidents.
3. Chemistry of OxadiazoleMoiety
Oxadiazoles are five-membered heterocyclic compounds that possess one oxygen atom and two nitrogen atoms in the ring system [12]. Depending on the position of heteroatoms (oxygen or nitrogen), there are different isomeric forms of oxadiazole moiety such as 1,2,3-oxadiazole, 1,2,4-oxadiazole, 1,2,5-oxadiazole, 1,3,4-oxadiazole (Figure 2).These chemical compounds are of the azole family with the molecular formula C2H2N2O. Among these isomers, 1,2,3-oxadiazole is unstable and ring-opens to form the diazoketone tautomer. However, 1,3,4-oxadiazole is a thermally stable aromatic molecule and plays a major role in developing new drug candidates with diverse biological activities such as anticancer, antiparasitic, antifungal, antibacterial, antidepressant, antitubercular and antiinflammatory, etc. [13].
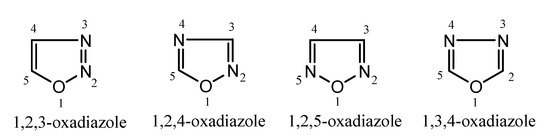
Figure 2. Chemical structures of oxadiazole isomers.
The electrophilic-substitution reaction is very difficult at the carbon atom in the oxadiazole ring because of the relatively low electron density on the carbon atom. However, the electrophilic attack occurs at nitrogen if the oxadiazole ring is substituted with electron releasing groups. Similarly, the oxadiazole ring is usually resistant to nucleophilic attack. However, the halogen-substituted oxadiazole undergoes nucleophilic substitution with the replacement of halogen atom by nucleophiles [14]. Although 1,3,4-oxadiazole ring system was known in 1880, significant studies were carried out regarding its chemistry, structure, physical properties and application of its various derivatives from 1950 (Table 1). 1,3,4-oxadiazole is a liquid with a boiling point of 150 °C. The percentage of C, H, N present in 1,3,4-oxadiazole is 34.29%, 2.88%, 40.00%, respectively [15].
Table 1. Bond angle and bond length of 1,3,4-oxadiazole moiety.
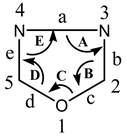 |
|||
|---|---|---|---|
| Angles | Bond Angle (°) | Bonds | Bond Length (Pm) |
| A | 105.6 | a | 139.9 |
| B | 113.4 | b | 129.7 |
| C | 102.0 | c | 134.8 |
| D | 113.4 | d | 134.8 |
| E | 105.6 | e | 129.7 |
The first monosubstituted 1,3,4-oxadiazoles were reported in 1955 by two independent laboratories. Since 1955, other research groups have performed the reactions of 1,3,4-oxadiazole and reported that it is a liquid that boils at 150°C. Ainsworth first prepared 1,3,4-oxadiazole (2) in 1965 by the thermolysis of ethylformate formyl hydrazone (1) at atmospheric pressure as depicted in Scheme 1 [16].
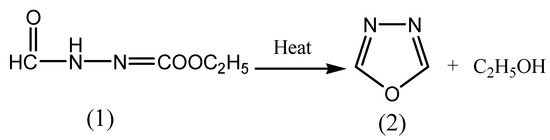
Scheme 1. Synthesis of 1,3,4-oxadiazole.
The 1,2,4-oxadiazole was synthesized first time in 1884 by Tiemann and Kruger. Most of the oxadiazole synthesis is based on heterocyclization of amidoxime and carboxylic acid derivatives or 1,3-dipolar cycloaddition of nitrile and nitrile oxide [17]. Microwave irradiation can also be applied in the heterocyclization of amidoximes and acyl chlorides/carboxylic acid esters in the presence of NH4F/Al2O3 or K2CO3 to produce corresponding oxadiazole derivatives [18]. Similarly, oxadiazole derivatives are produced by the reaction of aryl-nitrile with hydroxylamine hydrochloride to aryl-amidoxime inthe presence of a catalyst (MgO or CH3COOH or KF) under a microwave-assisted method. In the year 2017, Baykov et al. reported a study on the first one-pot synthetic procedure for the synthesis of 3,5-disubstituted-1,2,4-oxadiazoles (3) at room temperature from corresponding amidoximes (1) and carboxylic acids methyl or ethyl esters (2) in the presence of superbase medium NaOH/DMSOas presented in the Scheme 2 [19,20].
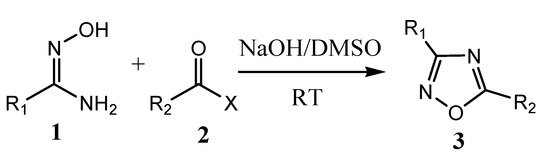
Scheme 2. Synthesis of 1,2,4-oxadiazole analogs. R1 = 4-methylphenyl, R2 = methyl or phenyl, X = methoxy or ethoxy.
Gorjizadeh et al. reported the efficient synthesis of a series of 1,3,4-oxadiazoles (3) from the cyclization–oxidation reaction of acyl hydrazones (1) with substituted aldehydes (2) by using 1,4-bis(triphenylphosphonium)-2-butene peroxodisulfate (BTPPDS) as an oxidant in a solvent-free medium under microwave irradiation (Scheme 3). The reaction was found to proceed smoothly under microwave irradiation within 25 min, whereas 12 h were required to complete the reaction under reflux conditions [21].
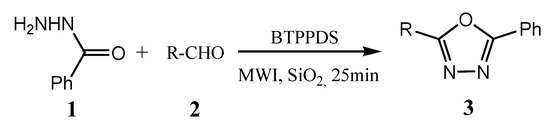
Scheme 3. Synthesis of a series of 1,3,4-oxadiazoles.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26041163
This entry is offline, you can click here to edit this entry!
