Nowadays, there is increasing interest in fast, accurate, and highly sensitive smart gas sensors with excellent selectivity boosted by the high demand for environmental safety and healthcare applications. Significant research has been conducted to develop sensors based on novel highly sensitive and selective materials. However, there are still great challenges, specifically in terms of selectivity, which raises the need for combining interdisciplinary fields to build smarter and high-performance gas/chemical sensing devices. This review is divided into two parts: In the first part, we cover the recent developments and limitations of chemresistive gas sensors and in the second part, machine learning is proposed as a potential approach to efficiently tackle these issues through pattern recognition algorithms. The review concludes that machine learning can be very promising in terms of building the future generation of smart, sensitive, and selective sensors.
- chemiresistive and FET sensors
- carbon materials
- 2D TMDCs
- metal/metal oxide
- density function theory (DFT)
- selectivity
1. Introduction
Today, the rapid expansion in automobiles and industries emerge as a serious threat to the environment and human health safety. Therefore, along with the strict implementation of industrial waste regulations, the demand for highly reliable and accurate sensors has become essential for human survival [1,2,3,4,5]. Over the past decades, several gas-sensing mechanisms have been reported, including resistive/chemiresistive [6], electrochemical [7,8] (amperometric and potentiometric), work function (Schottky diode, metal–oxide–semiconductor field-effect transistors (MOSFET) [9,10], etc.), optical (surface plasmon resonance (SPR)), and surface acoustic [11,12,13,14]. Among them, resistive type gas sensors have been extensively studied due to their small size, low power consumption, cheap and simple fabrication process [10]. To date, various materials have been developed and used for resistive-type sensing, such as carbon materials (graphene/reduced graphene oxide (rGO) [15,16,17,18], carbon nanotubes (CNTs) [9,19], carbon and graphene dots [20,21,22], three-dimensional (D) graphene [23,24], etc.), 2D transition metal dichalcogenides (TMDCs) [25,26], metal oxides (zinc oxide (ZnO) [27], tin oxide (SnO2) [28], titanium oxide (TiO2) [29], tungsten oxide (WO3) [30], indium oxide (In2O3) [31,32], nickel oxide (NiO) [33], iron oxide (Fe2O3) [34,35,36], etc.) [6,37,38], and noble metal catalysts (pallidum (Pd), platinum (Pt), gold (Au), silver (Ag), aluminum (Al), rhodium (Rh), etc.) [15,39,40,41].
Carbon materials possess a higher surface area and have the capability for trace-level molecule detection at room temperature (RT). However, they are less selective and demonstrate a lower recovery rate due to their high binding energy with the gas molecules [42,43]. On the other hand, metal oxides (MOx) are good candidates to detect a wide range of gas molecules at higher concentration levels with a relatively faster recovery rate. However, they require higher operating temperature (OT) to generate favorable oxygen adsorbents (O2−, O−, and O2−) on sensing surfaces [44,45,46].
For environmental safety and better monitoring of human health, there is an urgent demand for the development of a sensor with trace-level molecule detection, minimum drift, high sensitivity, fast response/recovery, and excellent selectivity under different environments (dry and humid). With the aim to build a sensor having such properties, researchers have focused on synthesizing novel and sensitive sensing materials (SMs) using different techniques including surface morphology change/modification [22,27,30,46], doping [47], composition/hybridization [15,26,30,44,48,49], p–n junction formation [50,51], and core–shell structures [52,53,54,55]. SMs synthesized via these methods certainly have a key impact in improving sensor performances. For instance, recently Wu et al. [39] reported a high-performance NO2 sensor at RT using boron (B)- and nitrogen (N)-doped 3D reduced graphene oxide hydrogel (RGOH). In comparison with pure RGOH sensors, the B- and N-doped RGOH sensors exhibited 38.9 and 18 times higher responses toward 800 ppb NO2, respectively. Additionally, the fabricated sensors showed good linearity, reversibility, fast response/recovery, and impressive selectivity. The higher sensing performances from B- and N-doped RGOH sensors at RT was ascribed to several factors, including the doping effects of B and N, 3D porous rGO architecture with the enlarged surface-to-volume ratio, pore filling, charge hopping, abundant disorder, oxygenated groups, and high electron mobility [39]. On the contrary, metal oxide gas sensors, which require higher operating temperature, can also show good sensing performance via metal catalyst doping and morphological modification. Recently, Sanger et al. [56] presented a highly sensitive transparent NO2 sensor using aluminum (Al)-doped ZnO (AZO) hollow nanofiber synthesized via the sputtering method. Their sensors displayed maximum sensitivity at an OT of 250 °C with a detection range (DR) from 0.5 to 10 ppm. The high sensitivity of the transparent sensors was attributed to the higher surface area of the hollow nanofibers and the high impact frequency of the trapped NO2 gas inside the hollow compared to the solid counterpart nanofibers [56]. Similarly, Li et al. [57] reported Pd–Au nanoparticles (NPs) decorated on SnO2 nanosheets (NShs) for formaldehyde and acetone detection. They demonstrated the temperature-dependent selectivity of fabricated sensors toward formaldehyde and acetone. The results determined effective detection of acetone (@250 °C) and formaldehyde (@110 °C) with responses of 6.6 (acetone) and 4.1 (formaldehyde) towards 2 ppm concentration, and their corresponding detection limits were noted as 45 ppb and 30 ppb, respectively. The enhanced response was attributed to the chemical sensitization of Au, the electronic sensitization of Pd, and the synergistic effect of Pd–Au bimetallic NPs [57].
Additionally, bimetals/bimetal oxide core–shell structures were also studied to obtain better sensing outcomes [40,41]. Most recently, Xu et al. [58] studied formaldehyde (HCHO) detection using bimetal Ag@Pt core−shell nanostructures (NSs) decorated on ZnO nanowires (NWs) by an inkjet printing method. Optimized (with Pt60 and Ag40 atomic ratio) sensors demonstrated maximum response on an OT of 280 °C with DR varying from 120 ppb to 2 ppm. They described how Ag@Pt core−shell NPs play a vital role as a catalyst during the HCHO detection process by dramatically enhancing the oxidation of HCHO molecules on the ZnO (NWs) surface. As a result, more electron release brings a higher HCHO sensing response for the ZnO-based gas sensor [58].
2. Chemical Gas Sensors: Achievements and Challenges
In this section, we focus on the SMs and discuss their structural properties for better sensing. Later, we highlight some computational studies on different kinds of SMs and the critical performance-enhancing factors. Finally, we discuss recent experimental advancements in three different kinds of gas sensors including carbon materials, 2D TMDCs, and MOx.
The development of a chemical gas sensor with fast response/recovery time, maximum sensitivity, minimum aging drift, excellent selectivity, and repeatability are major research concerns and targets. Typically, a sensing material is considered a promising candidate if it possesses a high specific surface area and a highly reactive crystal facet/site for specific gas molecule adsorption with maximum charge transfer [6,20,25,44]. It is well documented that bulk metal/MOx materials change their physical and chemical properties entirely when synthesized in micro/nanostructures (NSs). Even by varying the nanostructured morphologies, dramatic change in material properties can be expected. For example, Miller et al. [74] investigated the various defects points on the SnO2 NPs and NW surfaces using the ultra-high spatial resolution scanning transmission electron microscopy (STEM) combined with cathodoluminescence (CL) system to interpret their role in manipulating the band gap of a nanostructure. They studied four different material samples and found that SnO2 NW decorated with NPs through sputtering reveals a higher number of defects and thus may enhance the sensor response. However, they proposed that more in-depth investigations under different temperatures and gases (oxidizing/reducing) are required to understand the effect of defects on gas sensing [45,74]. Tuning of the surface energy and altering the band gap both can be vital for providing active sites to gas molecules [32,75,76,77,78]. Therefore, considerable research works are conducted to develop and explore various micro- (from thick film to highly porous 3D hierarchical structure) and nanostructures (nanoparticles (NPs), nanorods (NRs), nanotube (NTs), nanowires (NWs), and nanocapsules (NCps), etc.) with the aim to build SMs with maximum specific surface area and higher number of active sites. Until now, numerous SMs have been synthesized using physical and chemical routes. Figure 1 displays different types of SMs and their micro/nanostructures with advantages and drawbacks. It also illustrates the significance of hybridization/composition formation to improve the overall performance. In particular, it suggests that the decoration of metal/metal oxide NSs on all other types of materials is extremely vital for higher catalytic reaction, depletion region formation (p–n junction and Schottky barriers), to improve response and selectivity. The detailed discussion on different heterostructures (p–n junction, Schottky barriers, and catalyst decoration) and their corresponding performance enhancement mechanisms is not within the scope of this review article. Interested readers are referred to recently published review articles [51,52,79].
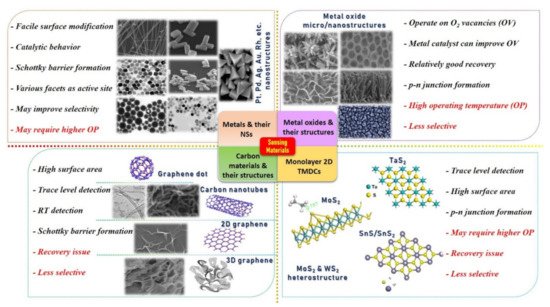
Figure 1. Different kinds of sensing materials with micro/nanostructures, their advantages (in black color), and limitations (in red color), indicating the need of hybridization/composition, doping, and p–n junction formation. In particular, the figure suggests that the decoration/doping of metal/hetroatom over the base materials will enhance the catalytic reaction for specific gases and form the charge accumulation depletion region to improve the sensing performances. Reproduced from multiple sources with permission from References [18,41,42,46,80,81,82,83,84,85,86,87,88,89,90,91]. Copyright 2016 Elsevier [18], copyright 2019 Elsevier [41], copyright 2016 Elsevier [42], copyright 2020 ACS [46], copyright 2020 [80], copyright 2019 Elsevier [92], copyright 2006 ACS [82], copyright 2007 ACS [83], copyright 2013 Elsevier [84], copyright 2014 Elsevier [85], copyright 2016 Elsevier [86], copyright 2018 ACS [87], copyright 2015 Elsevier [88], copyright 2014 Elsevier [89], copyright 2013 Nature [90], and copyright 2012 ACS [91].
Typically, SMs of a resistive-type gas sensor are composed of composite/hybrid materials, categorized as base and catalyst, and each has its role to play for better sensing performances. Base materials are usually responsible for providing a high surface area for gas molecule adsorption and a conductive pathway between two electrodes. This category includes microstructure thick films, thin films, porous films, 3D hierarchical structures, graphene, reduced graphene oxide, 3D graphene, and 2D transition metal dichalcogenides (TMDCs). On the other hand, metal/metal oxide catalysts, synthesized in nanostructures (NSs) and decorated over the base material, are used to enhance the reaction rate on the sensing surface for a specific gas. Therefore, a catalyst is an influential element in improving sensing performances, especially selectivity and response.
2.1. Computational and Experimental Research
2.1.1. Identifying Key Performance-Enhancing Factors through Computational Analysis of the Graphene, TMDCs, and Metal Oxides Sensors
Over the decades, great efforts have been devoted to understanding the behavior of the gas molecules on different atomic/molecular sites of the SMs through density function theory (DFT) calculations. It is suggested that adsorption energy, charge transform, and distance between gas molecule and SM surface are highly significant in defining the performance ability of a sensor [25,26,93]. Gas molecule adsorption site/facet and landing orientation have a great influence on the adsorption energy and maximum charge transfer. High adsorption energy and charge transfer indicate high sensitivity and selectivity towards a specific gas. For instance, Cui et al. [94] studied the layer-dependent sensing performance of phosphorene using computational and experimental investigations. Maximum adsorption energy was noted for NO2 and was confirmed through variations in band structure, signifying higher sensitivity of phosphorene towards NO2 molecules (see Figure 2A). Nevertheless, even for NO2, the charge transfer was not very significant. Therefore, the decoration or doping of metal catalyst over the base SMs is needed to considerably raise the charge transfer and adsorption energy for specific gas [94]. Varghese et al. [95] investigated the gas-sensing properties of boron (B)-, aluminum (Al)-, and gallium (Ga)-doped graphene using DFT calculations. They discovered that B-doped graphene behaved more stable in humidity environment than that of Al- and Ga-doped graphene and displayed adsorption energies of −0.375 eV and −1.450 eV for NO and NO2 molecules, respectively. Furthermore, they calculated maximum adsorption energies for Al (−3.474 eV)- and Ga (−3.050 eV)-doped graphene towards NO2 molecules in a dry environment [95]. Figure 2B shows a schematic illustration of NO2 molecule adsorption on doped graphene along with its density of state (DOS) for all types of graphene. A clear change in charge distributions at Fermi energy level can be seen for all types of graphene after NO2 molecule adsorption. Likewise, Wang et al. [96] investigated the adsorption of CO molecules on pure graphene, N-doped graphene, and Al-doped graphene, and their results showed maximum charge transfer of 0.2346 eV between CO molecule and Al-doped graphene surface [96].
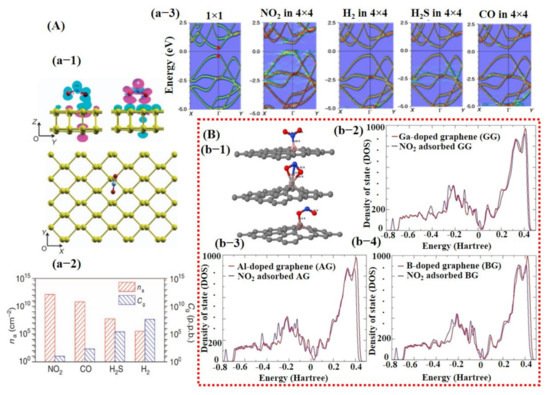
Figure 2. The computational investigation using DFT calculations: (A) DFT calculation for phosphorene: (a-1) shows adsorption of NO2 molecule on phosphorene surface with oxygen atoms pointing downward and the corresponding generated electron and hole clouds; (a-2) left axis indicates the histogram graph (red bars) with adsorption energies of different gases at fixed 20 ppb concentration; (a-3) displays the change in phosphorene band structure after adsorption of different gases including NO2, H2, H2S, and CO. A clear change on the energy level of conduction band can be observed with the NO2 adsorption on phosphorene surface, reproduced with permission from [94], copyright 2015 Nature. (B) DFT calculations of boron (B)-, aluminum (Al)-, and gallium-(Ga) doped graphene for NO2 detection: (b-1) illustrates a schematic for the NO2 molecule adsorption on all three kinds of graphene surfaces—clear and strong adsorption of NO2 molecule on Al-doped graphene can be seen (middle image); (b-(2–4)) show DOS for all kinds of graphene when exposed to NO2—Al-doped graphene displayed maximum change at Fermi energy level suggesting its higher sensing ability toward NO2 molecule (b-3). Reproduced with permission from [95], copyright 2016 Elsevier.
Similarly, iron (Fe)-doped single-layer and bi-layer graphene for CO, NO, SO2, and HCN adsorptions were explored by Tang et al. [97]. Fe-doped bi-layer graphene showed maximum adsorption for NO molecule and weakest for CO. Additionally, the semiconducting and magnetic behavior of SMs after gas molecule adsorption was also studied [97].
Besides graphene, 2D TMDCs and metal oxides have also been investigated using DFT calculations [26,98]. For example, a study by Yue et al. [99] considered the adsorption of different gas molecules including H2, O2, H2O, NH3, NO, NO2, and CO on pure MoS2 surface. They reported that all the gas molecules were weakly absorbed on MoS2 with less charge transfer, indicating the importance of doping/decoration of metal catalyst (see Figure 3A). They did not further continue their study with doping/decoration of a heteroatom. However, they proposed that the application of a perpendicular electric field promotes gas adsorption on the MoS2 surface [99]. More recently, Qian et al. [80] explored the effect of Au doping on 2D MoS2 monolayer sheets for C2H6 and C2H4 molecule detection. After Au doping, enhancement in adsorption energies and charge transfer between detection molecules and MoS2 monolayer was noted. The maximum adsorption energy and charge transfer were observed for the C2H4 molecule, finding it to be −0.952 eV and 0.309 e, respectively. Schematic diagrams are shown in Figure 3B of the Au@MoS2 surface with adsorbed gas molecules and DOS graphs. These techniques were not only studied in the computational domain but were also extensively investigated and verified through experiments.
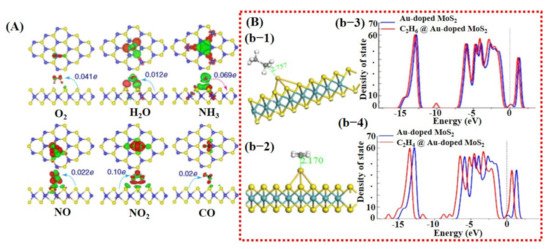
Figure 3. Computational analysis of MoS2 and Au-doped MoS2 for gas sensing using DFT calculations: (A) The charge transfer between pure MoS2 sheet and different target molecules; maximum charge transfer of 0.10 e can be seen for NO2, which is quite low, indicating the significance of heteroatom doping (reproduced with permission from [99], copyright 2013 Springer Nature). (B) The DFT results for Au-doped MoS2 sheet: (b-1) a schematic illustration of C2H4 and C2H6 molecule on Au-doped MoS2 with the corresponding bond length distance; (b-3,4) the DOS graphs for both the molecules before and after adsorption. A relatively larger change at Fermi energy level was found for C2H4, indicating better sensitivity of Au-doped MoS2 towards C2H4. Reproduced with permission from [80], copyright 2020 Front. Mater.
Saadi et al. [100] investigated the NO2 sensing mechanism on the WO3 surface, and they observed that the 001 facet of WO3 was more stable and suitable for NO2 sensing. Furthermore, they discussed the significance of oxygen vacancies on metal oxide surfaces and their role in the dissociation of NO2 molecules for the generation of more vacancies, and thus more active sites for the target molecule. DOS results displayed a change in charge distribution at Fermi energy level after NO2 adsorption, indicating the sensitivity of WO3 (001) surface towards NO2 molecules (Figure 2). The schematic illustration and reaction details/equations for NO2 dissociation in NO and again forming to NO2 are shown in Figure 4A. Bai et al. [101] reported both computational and experimental results of Al-doped ZnO NSs for CO sensing. Their experimental and simulated works were well matched and showed enhanced sensing performance towards CO when ZnO NSs were doped with Al (Figure 4B).
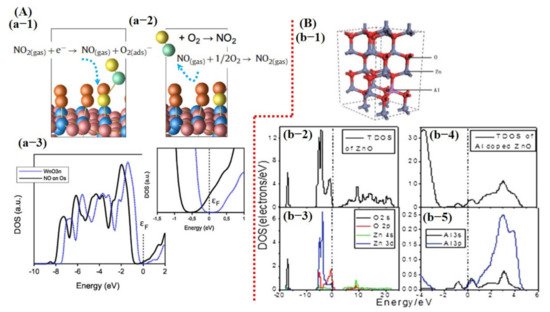
Figure 4. Computational study of metal oxide gas sensors: (A) NO2-sensing mechanism on WO3 surface and DOS graph before and after exposure of NO2: (a-1) The dissociation of NO2 molecule to NO when interacting with WO3 surface while leaving behind oxygen adsorbent. (a-2) The formation of NO2 from generated NO after reacting with one-half oxygen in the environment. This chain cycle significantly improves the oxygen adsorbent population consequently enhances the sensor response. (a-3) The DOS graph. A clear shift can be observed after NO exposure in the magnified image (reproduced with permission from [100], copyright 2014 Elsevier). (B) (b-1) A schematic image of Al-doped ZnO structure; (b-2) the change in Fermi energy level before and after Al doping in ZnO structure (reproduced with permission from [101], copyright 2013 RSC).
This entry is adapted from the peer-reviewed paper 10.3390/s21082877
