Among various immunotherapies, natural killer (NK) cell cancer immunotherapy using adoptive transfer of NK cells takes a unique position by targeting tumor cells that evade the host immune surveillance. As the first-line innate effector cell, it has been revealed that NK cells have distinct mechanisms to both eliminate cancer cells directly and amplify the anticancer immune system. Over the last 40 years, NK cell cancer immunotherapy has shown encouraging reports in pre-clinic and clinic settings. In total, 288 clinical trials are investigating various NK cell immunotherapies to treat hematologic and solid malignancies in 2021. However, the clinical outcomes are unsatisfying, with remained challenges. The major limitation is attributed to the immune-suppressive tumor microenvironment (TME), low activity of NK cells, inadequate homing of NK cells, and limited contact frequency of NK cells with tumor cells. Innovative strategies to promote the cytolytic activity, durable persistence, activation, and tumor-infiltration of NK cells are required to advance NK cell cancer immunotherapy. As maturing nanotechnology and nanomedicine for clinical applications, there is a greater opportunity to augment NK cell therapeutic efficacy for the treatment of cancers. Active molecules/cytokine delivery, imaging, and physicochemical properties of nanoparticles are well equipped to overcome the challenges of NK cell cancer immunotherapy.
- nanoparticles
- NK cell therapy
- cancer immunotherapy
- tumor microenvironment
- NK cell activation
1. Introduction
Cancer is the second cause of death worldwide and still needs much effort to cure the desperate disease [1]. In the past decade, immunotherapies modulating anticancer immune responses for cancer elimination have made a remarkable revolution in cancer treatments [2]. The 2018 Nobel Prize in Physiology or Medicine selected immunotherapy pioneers. More understanding of immune system-related cancer biology at the cellular and molecular levels has allowed cancer immunotherapy to be rapidly advanced for clinical applications. Subsequently, various cancer immunotherapies, including immune checkpoint blockades, chimeric antigen receptor (CAR) T-cell therapy, cytokine therapy, natural killer (NK) cell therapy, and cancer vaccines, are able to exhibit notable successes in the clinics [3][4][5][6][7][8][9]. However, immune-suppressive tumor microenvironment (TME), immuno-therapeutic resistance, immuno-therapeutic ignorance, and off-target toxicity, i.e., immune-related adverse effects (irAEs), permitted only a small percentage of patients to experience a positive response [10][11][12]. Substantial effort to overcome the limitation of cancer immunotherapy and further development of cancer immunotherapy strategies are needed to advance this effective strategy to treat cancers [13].
NK cell cancer immunotherapy using adoptive transfer of NK cells takes a unique position to target tumor cells that evade the host immune surveillance [14][15][16]. NK cells belonging to innate lymphoid cells are cytotoxic, play roles in producing cytokines and essential immunosurveillance for viral infection and cancers [17][18]. NK cells are mainly localized at epithelial surfaces and quickly responding to pathogen invasion to maintain tissue homeostasis [19]. The lack of antigen receptors in NK cells is different from T and B lymphocytes. A wide range of germline-encoded activating and inhibitory receptors of NK cells can be engaged by particular ligands displayed on various kinds of cells (Figure 1). NK cell’s selective cytotoxic functions killing disease cells are finely tuned by the signaling balance between the activating and inhibitory receptors [20]. For healthy cells, NK cells preserve tolerance towards surrounding normal cells. The tolerance is mainly controlled through inhibitory receptors such as killer immunoglobulin-like receptors (KIRs) and natural killer group 2A (NKG2A) recognizing self-major histocompatibility complex (MHC) class I molecules (Figure 1) [21]. In the process of NK cell education, the strength of these inhibitory receptor/ligand interactions also strongly correlates with the generation of functional NK cells. The activation of the “turn on” signal for the selective cytotoxic effect is involving several activation receptors, including natural cytotoxicity receptors (NCRs: NKp46, NKp44, and NKp30) and natural killer group 2D (NKG2D), whose ligands are mainly stress-inducible molecules UL16 binding proteins (ULBPs), MHC class I chain-related protein A and B (MICA/B). With the activation receptors, NK cells can selectively attack virally infected cells or cancer cells that are expressing downregulated MHC class I molecules through “missing self-recognition” and “induced self-recognition” (Figure 1) [22].
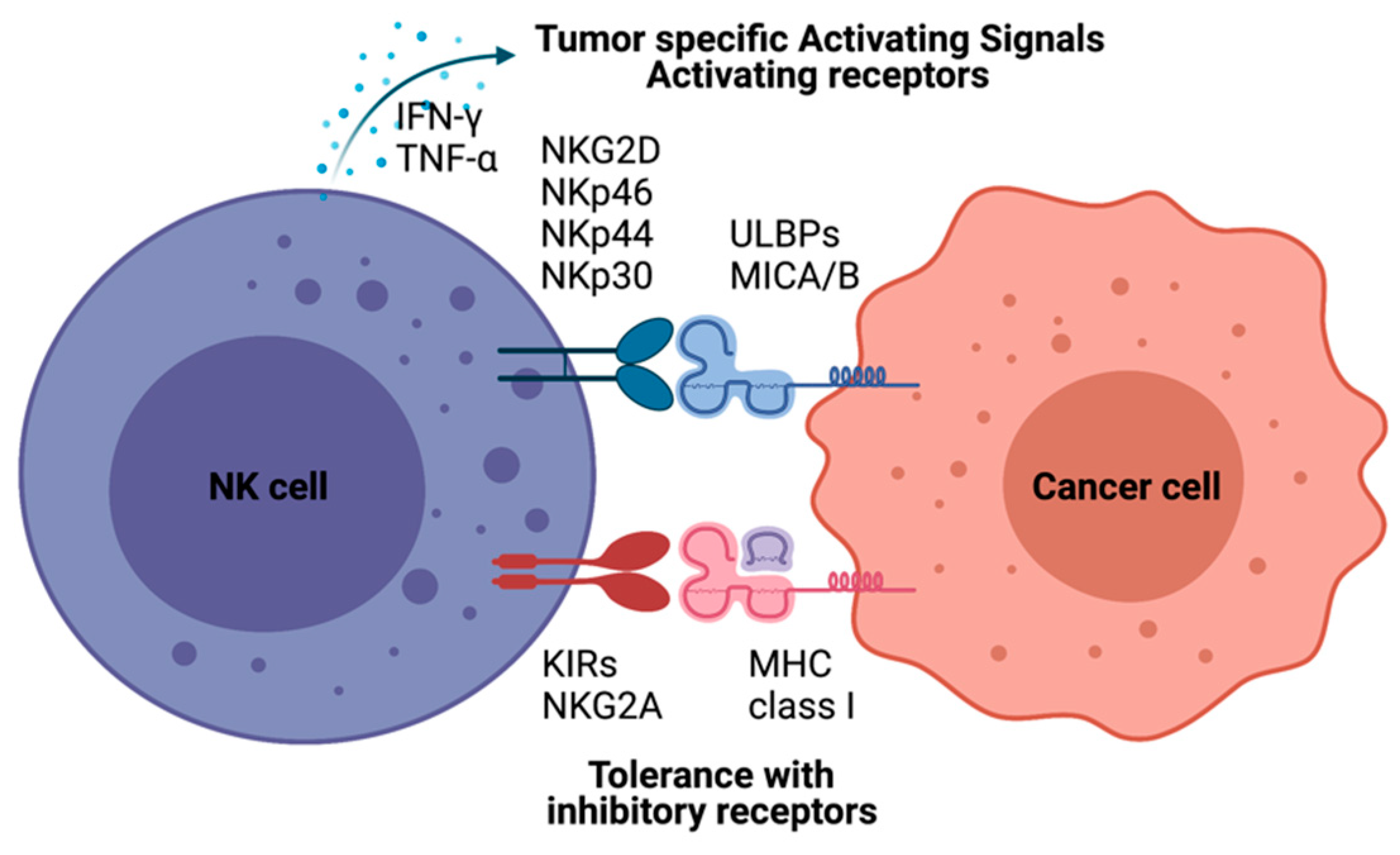
Figure 1. Natural killer (NK)-cell-mediated cytotoxicity. NK cell recognizes cells with NK cell receptors. MHC class I on target cells plays an inhibitory role binding to KIRs and NKG2A molecules resulting tolerance of NK cells as “self-recognition”. Otherwise, malignant cells inducing MICA/B, UL16 binding proteins (ULBPs), are detected by NK cells with NK-cell-activating receptors, including KG2D and NKRs. NK cells also have immune modulatory functions by producing IFN-γ and TNF-α to recruit other immune cells, such as dendritic cells and T cells.
These well-orchestrated selective cytotoxic functions of NK cells have prompted their use in many clinical trials to control tumor growth via their effector capacity. NK cell cancer immunotherapy has been considered an effective cancer treatment and a potent adjuvant to standard cancer treatment [23]. A total of 288 clinical trials are investigating NK cell immunotherapies to treat hematologic and solid malignancies in 2021 (www.clinicaltrials.gov). Those clinical trials using autologous NK cells, allogeneic NK cells, NK cell lines, and genetically modified NK cells have shown encouraging results in the response rate for various malignancies [24][25][26]. However, there are still considerable challenges in NK cell therapy to treat cancer patients. The TME structure and altered tumor immunogenicity lead to functional damage of NK cells and poor tumor trafficking and infiltration of NK cells into tumors [27]. Thus, various strategies to promote the expansion, cytolytic activity, durable persistence, activation, and tumor-infiltration of NK cells have been studied [28][29][30][31]. Recently, various multifunctional nanoparticles have been suggested to augment NK cell therapy for the treatment of cancers [32][33]. Active molecules/cytokine delivery, imaging, and physicochemical properties of nanomaterials are well equipped to overcome NK cell cancer immunotherapy challenges [34][35]. As maturing nanotechnology and nanomedicine for the clinical applications, there is greater opportunity for NK cell cancer immunotherapy. Here, we discuss recent NK cell adoptive cell transfer (ACT) clinical trials, challenges, and advances of nanoparticle-mediated NK cell therapeutic efficacy augmentation.
2. Challenges of NK Cell Cancer Immunotherapy
The primary reason for the therapeutic limitation of NK cell cancer immunotherapy was attributed to the immune-suppressive TME, low activity of NK cells, inadequate homing of NK cell adoptive transfer, and limited contact frequency of NK cells with tumor cells (Figure 2) [23][36].
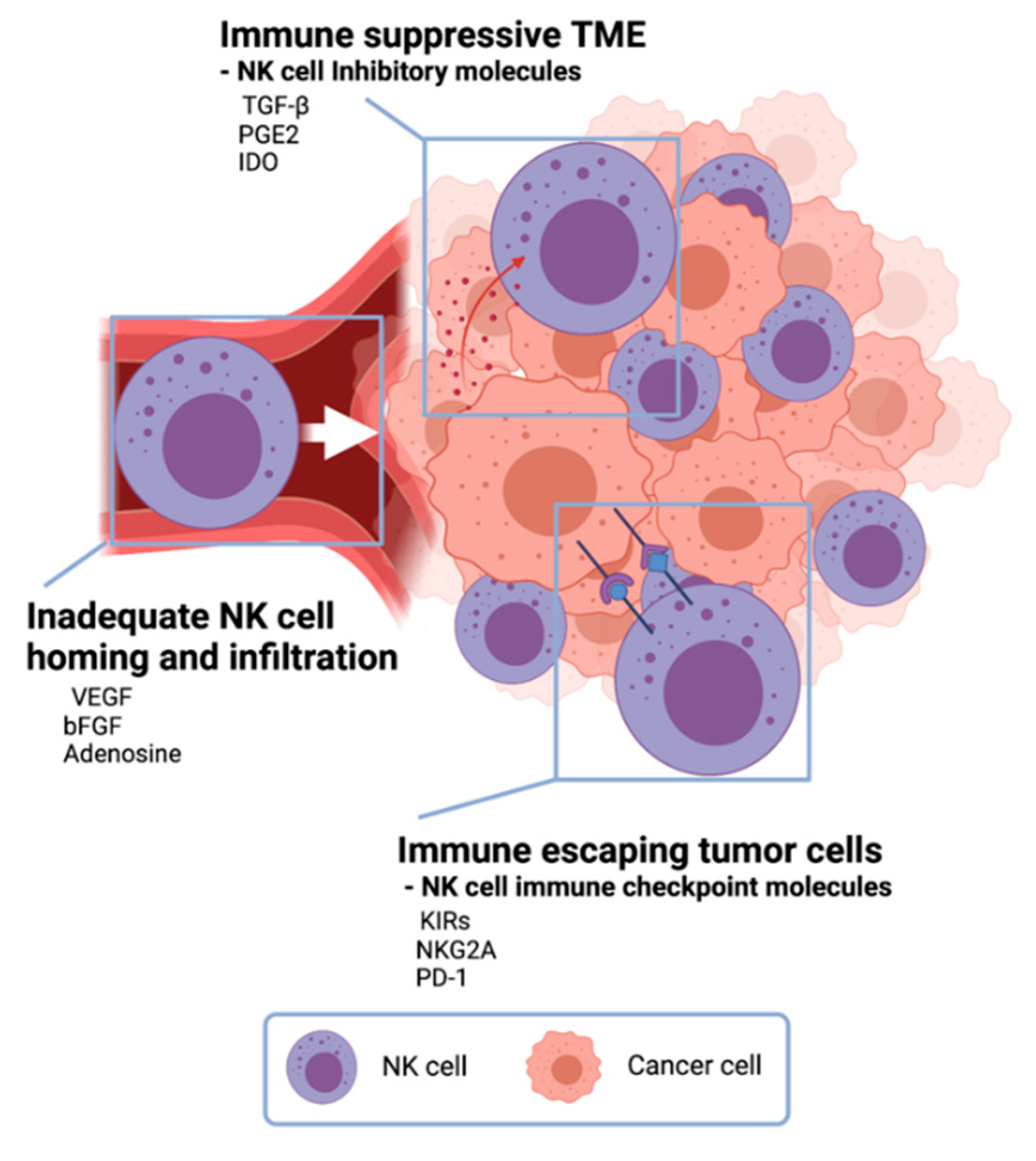
Figure 2. Challenges in NK cell cancer immunotherapy. In the tumor microenvironment, cancer cells secrete anti-immune molecules TGF- β, PGE2, and indoleamine 2,3-dioxygenase (IDO) to evade NK-cell-mediated tumor-cell lysis. Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and adenosine inhibit NK cells from homing to tumors resulting inadequate NK cell infiltration in tumors. Tumor cells express NK cell immune checkpoint molecules to escape from NK cells.
One of NK cell therapy’s limitations is the immunosuppressive effect of the TME (Figure 2) [6][37]. TME has unique environments built with various cancer cell-derived cytokines and following abnormal metabolic profiles. NK cells in the TME are changed to be low proliferation, decreased cytokine release, and downregulation of activation receptors [27]. Especially, there are immune-suppressive TME cytokines such as TGF-β, prostaglandin E2 (PGE2), and indoleamine 2,3-dioxygenase (IDO) [38]. Along with immune suppressive cytokines from tumor cells, regulatory T cells and myeloid-derived suppressor cells usually inhibit both the expansion and the function of effector NK cells with downregulating NK cell-activating receptors, IFN-γ, and cytolytic molecules [39]. To overcome this immune-suppressive TME, TME modulation involved with TGF-β, PGE2, and IDO cytokines and NK cell activation using IL-2, IL-12, IL-18, and IFN-γ have been actively studied for the augmentation of NK cell cytotoxicity against tumor cells in TME [40].
Tumors also exploit several defense mechanisms to limit NK cell homing and infiltration [41]. Deregulation of chemokine expression in the tumor is an important mechanism preventing NK cell infiltration and homing (Figure 2). Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) signaling on endothelial cells can repress adhesion molecule expression and prevent NK-cell infiltration [42][43]. So, there are many ongoing efforts toward improving tumor infiltration of adoptively transferred NK cells. The modification of NK cells with tumor-specific molecules and chemokine-chemokine receptor axis has been tried [44]. CCR5-CCL5 axis was induced to enhance NK cell infiltration in tumor tissue, and CXCR3 on NK cells also could interact with CXCL9, CXCL10, and CXCL11 from tumor cells [45][46]. The local delivery of those modified NK cells and catalytic molecules for migration was also studied to enhance NK cell therapy approaches. Ultrasound-mediated-, magnetic field-mediated-, and catheter-directed-NK cell delivery have demonstrated improved NK cell homing and infiltration [47][48][49].
Another critical challenge of NK cell adoptive transfer is the immune escape of tumor cells (Figure 2) [6][27]. Mutated tumor cells are expressing immune checkpoint molecules, rendering the immune system to be ineffective. Although the NK cell activation process involves more than one receptor–ligand interaction, NK-cell-mediated anticancer efficacy is often hindered by the low expression of NK cell activation receptor–ligand [50]. Subsequently, NK cell recognition in the tumor site is hampered due to the lack of NK–tumor contact. Highly effective immune cell engagers and specifically designed receptors effectively enhance the recognition and contact of NK cell-activating receptors [51]. Recently, CAR expressed NK cells showed an efficient cancer cell killing effect in CD19 positive leukemia and lymphoma cells [26]. Anti-CD19 CAR T cells were approved by the US Food and Drug Administration (FDA) in 2017. Despite the robust clinical response, the severe adverse effect was recognized [52]. Further studies for preventing immune escaping tumor cells are needed.
3. Conclusions and Future Outlook
NK-cell-based therapy has attracted significant attention in research on cancer treatment. Considering the tumor-specific cytotoxic function of NK cell cancer immunotherapy, NK cell cancer immunotherapy can be useful in many clinical cases. However, achieving meaningful therapeutic outcomes in the clinic is still challenging, owing to difficulties with the numerous immune-suppressive factors in the TME and poor NK cells’ homing and infiltration. Further development and refining of NK cell cancer immunotherapy are required. One promising direction would be the combinational NK cell cancer immunotherapy with other synergistic cancer therapies. Because NK cell infusion appeared to be safe and NK cells do not need a particular process of antigen recognition by antigen-presenting cells, there is the strength as a combination therapy regime. Utilizing distinct mechanisms of NK cells will be critical to have synergistic effects in combination with other cancer therapies, including chemotherapy, immune-modulating cytokines (IL-2 and IFN-γ) therapy, and immune checkpoint blockades immunotherapy. In advance of NK cell cancer therapy, nanoparticles will be a key tool, as they have recently shown great potential for augmenting the therapeutic efficacy of NK cell cancer immunotherapy. Engineered nanoparticles delivering various therapeutic agents, including antibodies, stimulatory cytokines, genes, or adjuvants, will enhance the NK cell activity, NK cell proliferation, and NK cell migration to tumor sites, thus markedly inhibiting tumor progression. The development of nanoparticles targeting the TME can also readily upregulate NK cell-activating ligands and stimulatory cytokines. Further, the medical imaging contrast effect of nanoparticles will allow image-guided NK cell cancer immunotherapy that can monitor NK cells and therapeutic prognosis. Indeed, the multifunctionality of nanoparticles interplaying between the immune system and tumors will allow the synergistic combinational anticancer effect, broaden the capacity of NK cell cancer immunotherapy, and contribute to developing safe and controlled NK cell cancer immunotherapies.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13040525
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021.
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489.
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086.
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73.
- Waldmann, T.A. Cytokines in Cancer Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10.
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK cell-cancer cycle: Advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 2020, 21, 835–847.
- Banchereau, J.; Palucka, K. Immunotherapy: Cancer vaccines on the move. Nat. Rev. Clin. Oncol. 2018, 15, 9–10.
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 73.
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300.
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164.
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16.
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561.
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196.
- Ljunggren, H.G.; Malmberg, K.J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat. Rev. Immunol. 2007, 7, 329–339.
- Parkhurst, M.R.; Riley, J.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive Transfer of Autologous Natural Killer Cells Leads to High Levels of Circulating Natural Killer Cells but Does Not Mediate Tumor Regression. Clin. Cancer Res. 2011, 17, 6287–6297.
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057.
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066.
- Huntington, N.D.; Cursons, J.; Rautela, J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454.
- Farag, S.S.; Caligiuri, M.A. Human natural killer cell development and biology. Blood Rev. 2006, 20, 123–137.
- Lanier, L.L. Natural killer cell receptor signaling. Curr. Opin. Immunol. 2003, 15, 308–314.
- Orr, M.T.; Lanier, L.L. Natural Killer Cell Education and Tolerance. Cell 2010, 142, 847–856.
- Wensveen, F.M.; Jelencic, V.; Polic, B. NKG2D: A Master Regulator of Immune Cell Responsiveness. Front. Immunol. 2018, 9, 441.
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7.
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123.
- Bachanova, V.; Sarhan, D.; DeFor, T.E.; Cooley, S.; Panoskaltsis-Mortari, A.; Blazar, B.R.; Curtsinger, J.M.; Burns, L.; Weisdorf, D.J.; Miller, J.S. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol. Immunother. 2018, 67, 483–494.
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553.
- Nayyar, G.; Chu, Y.; Cairo, M.S. Overcoming Resistance to Natural Killer Cell Based Immunotherapies for Solid Tumors. Front. Oncol. 2019, 9, 51.
- Muller, T.; Uherek, C.; Maki, G.; Chow, K.U.; Schimpf, A.; Klingemann, H.G.; Tonn, T.; Wels, W.S. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol. Immunother. 2008, 57, 411–423.
- Imai, C.; Iwamoto, S.; Campana, D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005, 106, 376–383.
- Cerwenka, A.; Lanier, L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016, 16, 112.
- Fehniger, T.A.; Cooper, M.A. Harnessing NK Cell Memory for Cancer Immunotherapy. Trends Immunol. 2016, 37, 877–888.
- De Lazaro, I.; Mooney, D.J. A nanoparticle’s pathway into tumours. Nat. Mater. 2020, 19, 486–487.
- Nam, J.; Son, S.; Park, K.S.; Zou, W.; Shea, L.D.; Moon, J.J. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019, 4, 398–414.
- Irvine, D.J.; Dane, E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334.
- Phung, C.D.; Tran, T.H.; Kim, J.O. Engineered nanoparticles to enhance natural killer cell activity towards onco-immunotherapy: A review. Arch. Pharmacal. Res. 2020, 43, 32–45.
- Oh, S.; Lee, J.H.; Kwack, K.; Choi, S.W. Natural Killer Cell Therapy: A New Treatment Paradigm for Solid Tumors. Cancers 2019, 11, 1534.
- Fang, F.; Xiao, W.; Tian, Z. Challenges of NK cell-based immunotherapy in the new era. Front. Med. 2018, 12, 440–450.
- Domogala, A.; Madrigal, J.A.; Saudemont, A. Natural Killer Cell Immunotherapy: From Bench to Bedside. Front. Immunol. 2015, 6, 264.
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front. Immunol. 2019, 10, 3038.
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869.
- Melero, I.; Rouzaut, A.; Motz, G.T.; Coukos, G. T-cell and NK-cell infiltration into solid tumors: A key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014, 4, 522–526.
- Vitale, M.; Cantoni, C.; Pietra, G.; Mingari, M.C.; Moretta, L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur. J. Immunol. 2014, 44, 1582–1592.
- Melder, R.J.; Koenig, G.C.; Witwer, B.P.; Safabakhsh, N.; Munn, L.L.; Jain, R.K. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat. Med. 1996, 2, 992–997.
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100.
- Susek, K.H.; Karvouni, M.; Alici, E.; Lundqvist, A. The Role of CXC Chemokine Receptors 1-4 on Immune Cells in the Tumor Microenvironment. Front. Immunol. 2018, 9, 2159.
- Li, F.; Sheng, Y.; Hou, W.; Sampath, P.; Byrd, D.; Thorne, S.; Zhang, Y. CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency. J. Immunother. Cancer 2020, 8.
- Alkins, R.; Burgess, A.; Kerbel, R.; Wels, W.S.; Hynynen, K. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro Oncol. 2016, 18, 974–981.
- Wu, L.; Zhang, F.; Wei, Z.; Li, X.; Zhao, H.; Lv, H.; Ge, R.; Ma, H.; Zhang, H.; Yang, B.; et al. Magnetic delivery of nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater. Sci. 2018, 6, 2714–2725.
- Su, Z.; Wang, X.; Zheng, L.; Lyu, T.; Figini, M.; Wang, B.; Procissi, D.; Shangguan, J.; Sun, C.; Pan, L.; et al. MRI-guided interventional natural killer cell delivery for liver tumor treatment. Cancer Med. 2018, 7, 1860–1869.
- Khan, M.; Arooj, S.; Wang, H. NK Cell-Based Immune Checkpoint Inhibition. Front. Immunol. 2020, 11, 167.
- Gleason, M.K.; Ross, J.A.; Warlick, E.D.; Lund, T.C.; Verneris, M.R.; Wiernik, A.; Spellman, S.; Haagenson, M.D.; Lenvik, A.J.; Litzow, M.R.; et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 2014, 123, 3016–3026.
- Brudno, J.N.; Kochenderfer, J.N. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019, 34, 45–55.
