1. Erectile Dysfunction and Cardiovascular Diseases
The Fourth International Consultation on Sexual Medicine has defined erectile dysfunction (ED) as the consistent or recurrent inability to attain and maintain penile erection sufficient for sexual satisfaction [
1] ED is classified as organic, psychological, or resulting from several simultaneous (mixed) factors, the most frequent form. Today, it is still problematic to accurately estimate the impact and the incidence of ED because of social, ethical, cultural, and religious reasons. Moreover, many men are convinced that sexual impairment is an inevitable feature of late age [
2,
3] leading to reduced and delayed medical advice.
The global mean ED prevalence ranges from 14% to 48%, with higher rates in the US and South East Asia than European rates [
1,
4]. In the U.S., at least 12 million men between 40 and 79 years of age have ED. In contrast, Italy has reported a prevalence of ED (complete and incomplete) of 12.8% and a significant incidence of age-related ED (2% between 18 and 30 years and 48% over 70 years) [
5]. Based on the Massachusetts Male Aging Study (MMAS) data, over a population range between 40 and 70 years, ED increased with age from 5.1% to 15% and from 17% to 34% for complete and moderate ED, respectively; mild ED remained stable at about 17% over the years. Furthermore, the prevalence of ED worldwide will be estimated to reach 322 million men by 2025 [
6]. Perhaps, this percentage is significantly underestimated.
Increasing evidence suggested an association between ED and cardiovascular diseases (CVD) [
7] with an increased prevalence of ED in cardiovascular patients and an increased prevalence of CVD in patients with ED [
8]. However, among clinical manifestations of atherosclerotic disease, ED usually proceeds by approximately five years the onset of coronary diseases, such as coronary disease, begins five years early the onset of carotid and peripheral disease with claudication [
9].
Performance anxiety and relationship issues are commonly recognized psychological causes of ED. Still, its prevalence is also related to several age-independent comorbidities, such as congestive heart diseases, atherosclerosis, blood hypertension, and other vascular disorders, psychiatric disorders (depression), endocrine disorders (diabetes, reduction of testosterone), neurological disorders, and concomitant other genitourinary disease related to surgery [
10].
2. Molecular Basis of Erectile Dysfunction
Several studies have shown that within the central nervous system, NO can modulate sexual arousal and erection [
31,
32,
33]. NO may act in several brain regions, and the paraventricular nucleus [
34], and an increase in NO production in this area has been demonstrated in experimental animal studies during copulation [
35]. NO may also mediate by Adrenocorticotropic hormone/a-Melanocyte Stimulating Hormone (ACTH/a-MSH) and 5-Hydroxytryptamine2C (5-HT2C) agonists’ actions, which, in turn, may trigger erections when injected into the ventricular brain system [
32]. Furthermore, the inhibitory effect of NOS inhibitors is not observed when these compounds are injected together with L-arginine, the substrate for NO [
36]. In the penis, the two principal sources of NO are the nonadrenergic, non-cholinergic nerves and the endothelium of penile arteries and cavernous bodies [
27,
37].
The primary electromechanical mechanism of contraction in VSMCs involves depolarization and opening of voltage-gated L-type Ca
2+ electromechanical channels, which allows the influx of extracellular Ca within the cell. The opening of the Ca-dependent potassium channels on the membrane leads to potassium outflux and hyperpolarization. Finally, the cytosolic Ca + + depletion causes cavernosal SMC relaxation leading to increased blood inflow through the helical arteries, sinusoidal filling and cavernosal dilation. At the same time, VSMC relaxation is related to the opening of K
+ channels (). Moreover, membrane potential changes due to increased K
+ efflux inactivate L-type Ca
2+ channels inhibit Ca
2+ influx. Thus, NO may also cause VSMC hyperpolarization. NO diffuses to SMCs, where it augments the formation of cGMP (nitric oxide-cyclic guanosine monophosphate (cGMP)), which acts as a second messenger [
27,
35,
36,
37,
38]. Then, cGMP that accumulates in SMCs is broken down by phosphodiesterase (PDE) enzymes [
28,
39], with PDE5 (phosphodiesterase-5) being the predominant isoform in the corpus cavernosum [
39] (). The same NO-cGMP pathway at the base of a healthy erectile function is the critical endothelium-derived pathway for vascular dilatation in the systemic and coronary circulation [
23,
40] which explains the tight pathogenic correlation between ED and coronary artery disease. Furthermore, NO mediates many of the endothelium’s antiatherogenic functions by blocking the expression of proinflammatory cytokines, chemokines, and leukocyte adhesion molecules [
41].
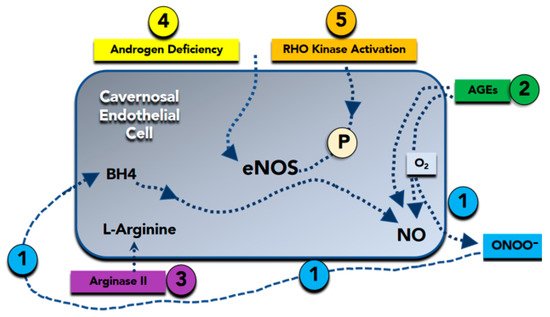
Figure 1. Molecular basis of nitric oxide (NO) reduction in erectile dysfunction. (Bullet 1) Superoxide (O2−) inactivates NO to form peroxynitrite (ONOO−). Peroxynitrite attacks and destroys tetrahydrobiopterin (BH4), a critical cofactor in NO synthesis. (Bullet 2) Advanced glycation end products (AGEs) promote superoxide formation, as well as directly inactivate NO. (Bullet 3) Arginase II degrades L-arginine, a substrate required for NO synthesis. (Bullet 4) Androgen deficiency reduces the expression of the endothelial isoform of NO synthase (eNOS). (Bullet 5) Rho-kinase reduces expression and phosphorylation of eNOS, which is required for its full activation. P = eNOS phosphorylation.
Therefore, loss of the biologic activity of endothelium-derived NO is coupled by other alterations in endothelial phenotype that further increase the propensity for systemic vasoconstriction, inflammation, and cellular proliferation [
42]. Furthermore, a reduction in NO availability due to impaired endothelial eNOS activity () and the subsequent increase in vasoconstriction causes a decrease in blood flow and oxygen supply, favoring free oxygen radical production. This inflammatory substrate favors, in turn, cavernous bodies fibrosis and progressive loss of erectile function [
19,
20,
21,
24].
2. Erectile Dysfunction and Cardiovascular Diseases
Among the different pathogenic mechanisms of ED (), vascular etiology is the most common cause [
43]. As a known symptom of atherosclerotic lesions, ED shares the same modifiable risk factors with coronary artery disease and peripheral artery disease, including hypertension, diabetes, dyslipidemia, cigarette smoking, obesity, and metabolic syndrome sedentary behavior.
Table 1. Etiologies of Erectile Dysfunction (ED).
ED is an independent risk factor for future cardiovascular events, being a potentially useful marker for cardiovascular disease [
44,
45,
46,
47]. ED commonly accompanies silent heart disease [
42,
43,
44] with an average time interval between the onset of ED and coronary heart disease by 2 to 5 years (class Ia) [
48,
49]. A recent metanalysis by Osondu and co-authors [
50] confirms an association between ED and subclinical cardiovascular diseases identified by different variables, such as endothelial dysfunction, with impaired flow-mediated dilatation, carotid intimal medial thickness, coronary artery calcification, ankle-brachial index, underscoring the importance of aggressive cardiovascular disease risk assessment and management in patients affected as the first onset by ED. Furthermore, a multicenter prospect cohort study [
51] in 1757 participants during a 3.8-year follow-up (interquartile range, 3.5–4.2) was recently published. Eight hundred and seventy-seven (45.8%) participants reported ED symptoms. Patients affected by ED were more likely to have diabetes mellitus and positive family history of coronary heart disease (CHD). Patients were also more likely to utilize β-blocker, antihypertensive, lipid-lowering, and antidepressant drugs. Over follow-up, a total of 40 CHD and 75 cardiovascular disease (CVD) hard events occurred in this cohort. In particular, a significantly greater proportion of patients affected by ED experienced hard events compared to those without ED (CVD hard events: 6.3% versus 2.6%,
p < 0.001; CHD hard events: 3.4% versus 1.4%,
p < 0.001). In the unadjusted Cox models, ED was a significant predictor of both hard CHD (hazard ratio, 2.5; 95% confidence interval [CI], 1.3–4.8), and CVD (hazard ratio, 2.6; 95% CI, 1.6–4.1) events. In the fully adjusted models, ED remained a significant predictor of hard CVD events (hazard ratio, 1.9; 95% CI, 1.1–3.4), whereas hard CHD events not.
Several explanations should be taken into account why ED is a precursor of CVD events. In this setting, Montorsi and co-authors [
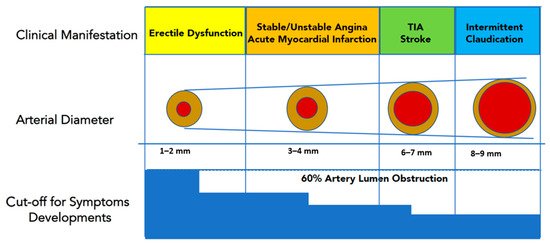
52] hypothesized that the smaller sized penile arteries (1–2 mm) would suffer earlier from atherosclerotic plaque burden, leading to arterial obstruction and flow compromise. Conversely, larger coronary (3–4 mm) or carotid arteries (5–7 mm) are affected later in the patient’s life span. Therefore, ED would represent early clinical evidence of a diffuse, systemic vascular disease, being “the tip of the iceberg” of preclinical cardiovascular disorders (). Another explanation [
53] is related to the increased arterial stiffness in the elderly, which can increase systolic blood pressure while decreasing diastolic blood pressure. The large-artery stiffness and subsequent systolic hypertension may force the pressure waves farther into smaller arteries leading to pudendal and penile arteries atherosclerosis. Abnormalities in the endothelial nitric oxide synthase (eNOS) production are also involved in endothelial dysfunction, which leads to ED and accelerated atherosclerosis. Indeed, ED has been associated with endothelial dysfunction of conduit vessels, increased coronary artery calcification, and silent angina independent of traditional cardiovascular risk factors [
53]. Furthermore, a chronic hypoxemic stimulus is an independent risk factor for the development of ED; this occurs, for example, in obstructive sleep apnea syndrome and chronic lung disease. Hypoxia determines an increase in vasomotor tone and causes the stimulus for vascular growth factors production, inhibiting the endothelium-dependent relaxation, and favoring corporal arterioles vasoconstriction.
Figure 2. Luminal narrowing due to atherosclerotic burden will manifest clinically earlier in penile arteries (smaller caliper) than in coronary, carotid, or iliac district (larger caliper). This explains why ED represents the “tip of the iceberg” of systemic atherosclerotic disease, and it is usually the first “alert sign” to manifest before a major cardiovascular event (Modified from Reference [
9]). TIA, transient ischaemic attack.
Traditionally, major traditional cardiovascular risk factors, such as diabetes, hypercholesterolemia, hypertension, and cigarette smoking, promote endothelial dysfunction and, ultimately, vasculogenic ED [
44,
47,
54,
55]. Diabetic ED (DED) is associated to an insufficient response to NANC nerve stimulus and an inability to vasodilate small arterioles of the penis. This results from reduced production of eNOS related to endothelial dysfunction and atherosclerotic disease [
19]. Epidemiologic data report that up to 75% of diabetic patients have a lifetime risk of developing ED [
10] and ED in diabetics is more common than retinopathy or nephropathy [
56]. Moreover, the clinical picture of ED is accelerated in diabetic patients: its onset occurs at an earlier age, presenting within ten years of the diabetic onset in more than 50% of patients with any type of diabetes [
57]. Histopathological analysis of cavernous bodies specimens from diabetic men with ED demonstrated ultrastructural changes in the cavernous arteries, cavernous smooth muscle, and impaired endothelium-dependent relaxation of the corporeal SMCs [
19]. In 12% of type 1 diabetic men, ED was the first symptom of diabetes [
58]. The presence of ED in diabetic patients could be a significant precursor of cardiovascular disease. Gazzaruso et al. [
59] found a higher prevalence of ED in diabetic patients with silent CHD than those without any evidence of myocardial ischemia. Moreover, ED was associated with higher major cardiovascular morbidity and mortality in diabetic patients with silent CHD.
The prevalence of hypertension in the ED population is higher than in people without ED [
60] and vice versa, a high prevalence of ED is generally observed in hypertensive patient populations [
61]. Both antihypertensive drugs and hypertension alone can deteriorate the erectile function. High blood pressure is characterized by increased peripheral sympathetic activity, which maintains an elevated vasoconstrictor tone and decreases the endothelium-dependent vasodilation in arteries leading to consequent alterations in vessel architecture and diminished dilatory capacity; moreover, vascular remodeling could also occur at the corporal level, progressing to ED by altering mechanical properties of erectile tissue [
61]. ED is usually associated with longer duration and more severe hypertension. In hypertensive rats, the impairment of cavernosal endothelium-dependent and NO donor-induced relaxations also occurred before systemic vascular alterations are manifested [
62], suggesting that erectile tissue is an early end-organ target for developing endothelial dysfunction in this patients’ cohort.
The association between ED and CVD in patients with preexisting cardiac conditions is complex and requires the interaction of urologists and cardiologists. In patients affected by ED, baseline investigations should include the assessment of ED using validated questionnaires, such as the International Index of Erectile Function in order to assess ED severity. Because episodic sexual activity could trigger acute cardiac events in specific CVD patients, exercise assessment is a critical step in managing ED. Evaluation should be done before a patient can be counseled about the safety of sexual intercourse and the use of pro-erectile drugs. The stress of sexual performance on the heart corresponds to a medium level of physical activity, as to complete 4 min of the standard Bruce treadmill test, up to 4–5 Metabolic Equivalents (METs). In general, if a heart patient can achieve this during exercise symptom-free level, should be able to have sex without cardiovascular problems. However, patients must be stratified by their likelihood of CVD events or their mortality both during and immediately after sexual activity.
Low-risk patients can safely perform sexual activity and should receive ED treatment. In this group are included men that were successfully revascularized, or men with asymptomatic controlled hypertension, mild valvular disease, and class I and II heart failure according to the New York Heart Association (NYHA) classification. High risk category indicated men with unstable angina, uncontrolled hypertension, NYHA class IV heart failure, myocardial infarction within two weeks without intervention, high-risk arrhythmia, symptomatic hypertrophic cardiomyopathy, and moderate to severe valve disease. These men should defer sexual activity until the cardiac condition has been stabilized, receive intensive risk-factor correction. Men not included in these two groups are considered as patients with indeterminate risk. Those patients should be reassessed using the stress test and, in turn, be reassigned to a low- or high-risk category [
63].
Phosphodiesterase-5 Inhibitors in Patients affected by Coronary Artery Disease PDE5i have shown to effectively improve erectile function when assumed on demand and are now considered first-line pharmacotherapy for treatment of ED. PDE5i have an excellent safety profile and can be administered to CVD patients. The most widely prescribed PDE5i approved for the treatment of ED are Sildenafil citrate (Sildenafil), Tadalafil, and Vardenafil that have proven efficacy in treating erectile dysfunction and also are currently prescribed on pulmonary arterial hypertension (PAH). PDE5i shown some differences in their biochemical properties, pharmacokinetic profiles, and clinical performance. In particular, Tadalafil absorption does not seem to be influenced by the intake of fatty meals or alcohol; the peak of serum concentration is reached about 2 h after the dose instead of 1 h with the other two PDE5i, moreover half-life has a duration of 17.5 h compared with 3.7 h for sildenafil. Furthermore, Tadalafil administration improves erectile function up to 36 h post-dose. The theoretical impact of these pharmacokinetic properties is that spontaneous sexual activity can be more easily restored by chronically administering this drug. On the other hand, the prolonged half-life results in greater long-term adverse effects (such as headache) than other PDE5i. However, there is no consensus on which drug is most recommended for ED treatment. Patient’s choice and physician’s judgement must be considered when prescribing a PDE5i.
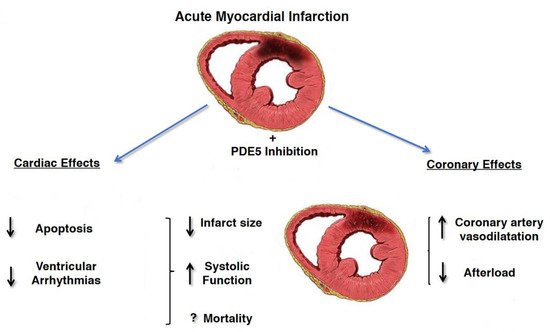
The beneficial effects of PDE5i on the cardiovascular system are supported by numerous animal and human studies showing sustained improvement in hemodynamics parameters including arterial stiffness, flow-mediated dilation, and peak systolic velocity, even after discontinuation. These findings may be due to the positive effects of PDE5i on endothelial function and in particular on vasodilation, thrombosis and inflammation. In fact, PDE5i improve erectile function by increasing the availability of nitric oxide in the penis and its vascular system, resulting in vasodilation and increased blood flow. PDE5i could benefit cardiovascular disease because phosphodiesterase-5 is also found in other parts of the body, including the pulmonary and systemic vascular systems and in hypertrophic myocardium. PDE5i are in fact used in primary pulmonary arterial hypertension with reversible pulmonary arterial resistance. In addition, PDE5i appear to protect the myocardium through complex pathways involving nitric oxide, cyclic guanosine monophosphate, protein kinase G, extracellular signal-regulated kinase, B-cell lymphoma protein 2, and Rho kinase inhibition. In animal models of acute myocardial infarction, PDE5i consistently reduced the size of the infarct indicating cardio-protection. PDE5i also promote reverse remodeling and reduce myocardial apoptosis, fibrosis and hypertrophy [
49,
64,
65]. Those cardiovascular beneficial effects were stronger in patients with prior myocardial infarction (MI) and were associated with reduced incidence of new MI, raising the possibility that PDE5is could prevent both complications post-MI and future cardiovascular events [
66] () In particular, in a Swedish nationwide cohort study, 43.145 men <80 years of age without prior MI, or cardiac revascularization, hospitalized for MI during 2007–2013 were evaluated for the risk of death, MI, cardiac revascularization or heart failure after treatment with PDE5i or alprostadil [
67]. Men with, compared with those without treatment for ED, had a 33% lower mortality (adjusted Heart Ratio (HR) 0.67 (95% CI 0.55 to −0.81)), and 40% lower risk of hospitalization for heart failure (HR 0.60 (95% CI 0.44 to 0.82)). There was no association between treatment with alprostadil and mortality. The adjusted risk of death in men with 1, 2–5 and >5 dispensed prescriptions of phosphodiesterase-5 inhibitors was reduced by 34% (HR 0.66 (95% CI 0.38 to 1.15)), 53% (HR 0.47 (95% CI 0.26 to 0.87)), and 81% (HR 0.19 (95% CI 0.08 to 0.45)), respectively, when compared with alprostadil treatment, suggesting a dose-dependent effect of PDE5i. Another study, enrolled 5956 pts, aged 40–89 years, with a prior history of type 2 diabetes with high attendant cardiovascular risk [
68] to describe the potential cardioprotective action of on-demand PDE5i administration in overall mortality. Diabetic pts treated with PDE5i was associated with a significant lower rates of incident MI (incidence rate ratio 0.49 to 0.80),
p < 0.0001) with lower all-cause mortality and a lower proportion of death (25.7% vs. 40.1% deaths;
p = 0.001) compared with non-users. This lower mortality risk in those taking a PDE5i persisted after adjustment for known risk modifiers including previous stroke, previous MI, age, estimated glomerular filtration rate (eGFR), CVD, hypertension and use of cardioprotective agents, such as β-blockers and statins. In addition, in a subgroup analysis of patients with history of MI or an incident MI during the study period, PDE5i use was associated with significantly lower mortality risk. A recent nationwide observational cohort study [
69] enrolled 18,542 men with stable CHD; 16,548 men were treated with PDE5i and 1994 pts were treated with alprostadil. The mean follow-up was 5.8 years, with 2261 deaths (14%) in the PDE5i group and 521 (26%) in the alprostadil group. Moreover, men with PDE5i treatment showed lower long-term risk of all-cause and cardiovascular mortality, MI, heart failure, and cardiac revascularization after adjustment for potential confounders, including marital status and length of education.
Figure 3. Cardioprotective effects of PDE5i in patients affected by acute myocardial infarction. Reduction of infarct size related to decrease myocardial cells’ apoptosis, decrease in afterload due to increase vasodilatation and increase in systolic function has been demonstrated (Modified from Ref. [
70]).
3. Guidelines for Therapeutic Management of Erectile Dysfunction
The American Urological Association and the European Urology Association have recently published new guidelines on the management of erectile dysfunction [
71,
72], to offer a high-quality resource for assisting clinicians and patients in understanding the benefits and risks/burdens of the various management strategies for ED. The first management strategy consists of lifestyle modifications, including diet changes and increased physical activity, which improve overall health and may improve erectile function (
Moderate Recommendation; Evidence Level: Grade C). The second recommendation is that men affected ED should be informed regarding the treatment option of oral phosphodiesterase type 5 inhibitor (PDE5i), unless contraindicated. (
Strong Recommendation; Evidence Level: Grade B). In addition, clinicians should provide instructions to patients to maximize drugs benefit/efficacy, including the fact that there is a non-linear dose-response effects across PDE5i medications, and on-demand dosing versus daily dosing for tadalafil appears to produce the same level of efficacy (
Strong Recommendation; Evidence Level: Grade C).
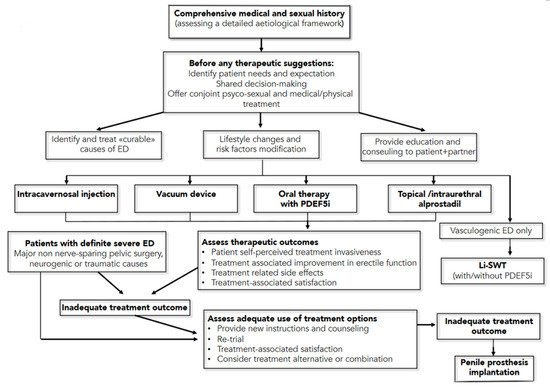
Moreover, the dose of PDE5i should be titrated to provide optimal efficacy (Strong Recommendation; Evidence Level: Grade B). The use of nitrate-containing medications, combined with a PDE5i, can cause severe hypotension and anginal symptoms in patients affected by CAD. As such, men taking nitrates should not use PDE5i medications. Patients should also be informed regarding a vacuum device’s treatment option to treat arteriogenic erectile dysfunction (Moderate Recommendation; Evidence Level: Grade C). Men with ED should be informed regarding the treatment option of either intraurethral (IU) alprostadil or intracavernosal injection (ICI—with alprostadil, papaverine, phentolamine, and/or atropine) and an in-office training of both treatments options is recommendable (Conditional Recommendation; Evidence Level: Grade C). For men with ED, low-intensity extracorporeal shock wave therapy, platelet-rich plasma therapy and intracavernosal stem cell therapy must still be considered investigational (Conditional Recommendation; Evidence Level: Grade C). For young men with ED and focal pelvic/penile arterial occlusion and without documented generalized vascular disease or veno-occlusive dysfunction, penile surgical arterial reconstruction should be considered (Conditional Recommendation, Evidence Level: Grade C), while penile venous ligation by surgery is not (Moderate Recommendation, Evidence Level: Grade C). Patients should be informed regarding the treatment option of penile prosthesis implantation, including discussing benefits and risks/burdens (Strong Recommendation, Evidence Level: Grade C). A summary of these guidelines’ recommendation is reported in .
Figure 4. Management Algorithm for erectile dysfunction (from European Association of Urology Pocket Guidelines 2020, p. 222). ED = erectile dysfunction; PDEF5i = phosphodiesterase type 5 inhibitors; Li-SWT = low-intensity shockwave treatment.