1. Plant Volatile Organic Compounds
Plant volatile organic compounds (VOCs) are specialized metabolites, which have been described from more than 90 plant families [
1,
2]. A wide diversity of compounds are emitted from leaves, flowers, fruits, bark, and roots, as well as from specialized storage structures, e.g., glands [
1,
2]. VOCs are lipophilic liquids and have low molecular mass (<300 Da) with high vapor pressures. Plant VOCs are derived from various biosynthesis pathways and are often classified into significant groups, including phenylpropanoids, fatty acids, amino acids, and terpenoids [
3].
VOCs are primarily intended to protect plants against pathogens and herbivores or to provide a reproductive benefit in attracting pollinators and seed dispersers [
3,
4,
5,
6,
7]. Plant VOCs have attracted considerable scientific interest for commercial applications, e.g., decorative cosmetics, shampoos, and fine fragrances, as well as for their potential benefits for human health [
8,
9,
10].
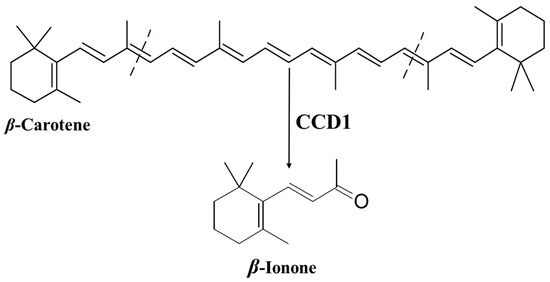
Ionone is a ketone with a monocyclic terpenoid backbone made up of 13 carbon atoms. The name “ionone” is derived from “iona” (Greek for violet), which refers to the violet odor, and “ketone,” which refers to the structure of the ionone [
11,
12]. β-Ionone is the 9,10 and 9′,10′ cleavage product of β-carotene () [
13,
14,
15,
16].
Figure 1. Production of β-ionone from β-carotene by carotenoid cleavage dioxygenase 1 (CCD1).
It is a common aromatic volatile found in various tissues in many plant species, e.g., in the essential oil (EO) of
Osmanthus fragrans, Petunia hybrid, and
Rosa bourboniana, and in fruits and vegetables, including carrot, tomato, melon, raspberry, apricot, plum, apple, and fig [
14,
17,
18,
19,
20,
21]. β-Ionone has become quite attractive for commercial applications and in medical and pharmaceutical industries [
22,
23,
24,
25,
26,
27,
28].
2. Occurrence of β-Ionone
Headspace solid-phase microextraction (HS-SPME) was used to obtain the EO of
Osmanthus fragrans, which was then analyzed by gas chromatography–mass spectrometry (GC–MS) at four different stages of flowering. The primary chemical components of the EO obtained from
O. fragrans were β-ionone,
α-ionone, and other VOCs, e.g., linalool and nerol [
29]. Capillary GC–MS with olfactometry analysis was used to determine the volatile profile of samples of
Thymus vulgaris EO. The compounds with the highest aroma impact for
T. vulgaris were β-ionone, borneol, terpinyl acetate, and β-damascenone [
30]. The EO of the leaves of
Lawsonia inermis L. (henna) was found to contain β-ionone as one of its major components [
31]. The EO of the genus
Rosa (Rosaceae) is commercially crucial in the fragrance and food industries [
32,
33,
34]. Honarvar et al. [
35] reported that EOs of fresh and dried flowers of Musk Rose contain approximately 2% of β-ionone of the total chemical constitutes. In the EO obtained from fresh aerial parts of
Viola tricolor (Violaceae), Anca et al. [
36] identified 35 VOCs, and β-ionone constituted around 2% of the total. The chemical composition of the EO from different stages of
Medicago marina was analyzed, and β-ionone was one of the main constituents of the flowering samples (6%) but increased during the vegetative stage (14%) [
37]. β-Ionone was also determined at different percentages in the EOs of Moroccan herbs, namely
Myrtus communis,
Cistus ladanifer, and
Montpellier cistus [
38]. In contrast, in the EO of maca (
Lepidium meyenii), β-ionone was found to be a minor component [
39] ().
Figure 2. β-Ionone as a fragrance compound in the essential oils of various plant species.
The aroma of fruits and vegetables is determined by unique combinations of VOCs, including β-ionone (), although different fruits, roots, and vegetables often share many aroma characteristics. Yahyaa et al. [
15] analyzed the VOC composition in the roots of various carrot genotypes with different colors (orange, purple, white, and yellow). They found that only orange and purple carrot roots accumulate β-ionone; white and yellow carrot roots do not [
15]. β-Ionone was also identified in the chemical compositions of EOs of
Daucus carota subsp.
sativus [
40]. Guillot et al. [
41] characterized the aromatic profile of several apricot cultivars (Iranien, Orangered, Goldrich, Hargrand, Rouge du Roussillon, and A4025) by using HS-SPME. In the apricot, several VOCs, including β-ionone, were recognized by HS-SPME–GC–Olfactometry methods as responsible for its aromatic notes [
41,
42,
43,
44]. Tomato fruit contains a complex mixture of VOCs and non-volatile compounds that contribute to the fruit’s overall aroma and taste, and β-ionone is involved in tomato’s flavor [
45]. Similarly, β-ionone was found to be a significant VOC in melon and watermelon [
14,
45]. Among stone fruits, in the plum (
Prunus domestica), SPME–GC–MS analysis of the VOCs revealed that β-ionone is one of the characteristic odor active compounds in fresh plums, with concentrations far above the odor threshold [
20,
46]. The carotenoid-derived volatile β-ionone plays an essential role in forming green and black tea flavors due to its low odor threshold [
47]. Moreover, this volatile, together with other VOCs, e.g., linalool and eugenol, contributes to the “spicy” character of hops (
Humulus lupulus L.), which is considered to be a desirable attribute in beer, associated with “noble” hop aroma [
48] ().
Figure 3. β-Ionone as an aroma compound found in some representative fruits and vegetables.
β-Ionone is an essential contributor to fragrance in the flowers of
Boronia megastigma [
49], in petunia (
Petunia hybrida) [
50], and in the yellow and red
Lawsonia inermis L. (henna) flowers [
51] (). Sweet osmanthus (
Osmanthus fragrans) is one of China’s ten most well-known flowers, and it is recognized as both an aromatic plant and an ornamental flower, with β-ionone as a major aromatic component [
29,
52,
53]. The flowers of the mangrove plant,
Nypa fruticans, are pollinated by various pollinators, including birds, bats, and insects. The floral scent chemistry of
N. fruticans is composed mainly of carotenoid derivates: β-ionone,
α-ionone, dihydro-β-ionol, and dihydro-β-ionone [
54]. These floral scents might be a critical factor in attracting pollinators.
Acacia farnesiana flowers’ volatile components contain β-ionone as one of the main components [
55]. β-Ionone is also the main volatile in the
Thevetia peruviana flowers [
56].
Figure 4. Representative flowers that accumulate β-ionone as a fragrance compound.
5. Microbial Production and Biotransformation of β-Ionone
Currently, the production of β-ionone is mainly dependent on chemical synthesis. The extraction of β-ionone from various plant species is limited, as it is for several other plant natural compounds, by low recovery rates and the complexity of the mixtures obtained from plants. Furthermore, the total chemical synthesis of β-ionone is economically impractical. As a result, one appealing alternative is to express the specific plant biosynthetic pathway genes in microbial hosts such as
Saccharomyces cerevisiae or
Escherichia coli, and then engineer the metabolic pathway/host to improve metabolite production [
89,
90]. The expression of heterologous genes using high-copy-number plasmids is a widely used approach for achieving high titers of secondary metabolites in biofactories. In most cases, however, this technique is unsuccessful because it necessitates selective media use, causes genomic instability, and creates a high metabolic burden on the cell [
91]. Genomic integration of expression cassettes, on the other hand, is a more convenient strategy because the resulting recombinant strains are generally more stable, and their gene expression is more controllable [
92,
93]. However, the small number of selection markers, integration sites, and genetic arrangements impedes strain design, as the acceptable gene dosage (copy number) for construction is restricted. CRISPR/Cas9 technology advancement in lees minimizes the need for selection markers, particularly when the transformation of
S. cerevisiae has proven to be highly successful [
94,
95].
Various high-value plant natural compounds, such as pharmaceutical compounds (e.g., artemisinin), fragrance (e.g., nootkatone), biofuels (e.g., ethanol and isobutanol), and food chemicals (e.g., vanillin and resveratrol), were produced as end products in microbial systems [
96]. Because of the rapid growth of microorganisms compared to plants, the accessibility of genetic manipulation, and the well-established metabolic engineering tools developed to be used in the microbial system, microbial production offers many advantages over each field and plant cell cultivation. This system can also produce much purer desired product from cell cultures than is obtainable from plant tissues or plant cultures, allowing for more straightforward (and “greener”) purification strategies. The functional reconstruction of specific plant biosynthetic pathway genes and enzymes in the microbial system, as well as the application of microbial biosynthesis for the industrial production of essential compounds, remain difficult tasks [
96,
97,
98,
99].
In addition to a comprehensive understanding of the biochemical pathway of interest, the microbial industrial production of the compound aims to provide information about the interaction between the pathway members (for example, substrate channeling, protein–protein interactions, side reactions), as well as precursor availability, high growth, production capacity, etc. Because of the extended plant growth cycle and low concentration of β-ionone, its direct extraction from plants affords the terpenoid’s low yields, insufficient to meet demand [
100,
101]. In fact, the annual industrial production of β-ionone is approximately 4000–8000 tonnes, and the demand is rapidly increasing [
102]. In particular, β-ionone and its derivatives have attracted great interest for their commercial application in decorative cosmetics, shampoos, fine fragrances, and toilet soaps [
22].
One of the first platforms used for β-ionone production was
S. cerevisiae, reaching titers in the range of 0.2 to 5 mg/L [
102,
103]. Recently, Werner et al. [
104], in two-phase fermentation in shake flasks, reported 180 mg/L of β-ionone production using a β-carotene hyper-producer strain. Nevertheless, this specific yeast strain has poor performance under industrial fermentation conditions [
105]. β-Ionone production in
E. coli has also been attempted. Zhang et al. [
106] reported titers of 32 mg/L β-ionone in shake flasks and 500 mg/L in bioreactors. Instead, using the oleaginous yeast
Yarrowia lipolytica, the authors of [
90] obtained titers of 60 mg/L in shake flasks and 380 mg/L of β-ionone in a fermenter. More recently, López et al. [
95] developed several recombinant
S. cerevisiae strains capable of producing increasing amounts of β-ionone (33 mg/L) and its precursor, β-carotene (33 mg/L), in the culture medium after 72 h cultivation in shake flasks.