Osteosarcoma (OS) is a high-grade malignant tumor of bone composed of mesenchymal cells (malignant osteoblasts) that is able to produce an immature woven bone and osteoid matrix.
- osteosarcoma
- comparative oncology
- microRNA
- Oncogeneses
1. Introduction
MicroRNAs (miRNAs) are highly conserved non-coding RNAs of ~22 oligonucleotides that exert a primary modulatory role on gene expression and can regulate more than 60% of the genome post-transcriptionally. They have been characterized in 61 tissues [1] and their presence has been discovered in 12 human body fluids [2]. The first descriptions of the roles played by microRNAs date back to the 1980s when they were identified by Chalfie in 1981, and, over the years, the numerous studies applied to oncology have shown how their deregulation can play a significant role in the etiopathogenetic mechanisms of some of the most common forms of cancers, both in humans and animals. The comparison of the expression of microRNAs among neoplastic and normal cells has further established their role in the oncogenesis of many tumors by means of interaction and promotion mechanisms on target genes [3]. Moreover, the miRNAs alterations often correlate with a more aggressive tumor biological behavior, making these molecules potential prognostic and therapeutic targets [4].
Comparative oncology is a field of study that assesses the cancer risk and tumor progression across species. The canine model is ideally suited for translational cancer research. Some histological and clinical characteristics of numerous canine tumors are strongly similar to those of the corresponding tumors in humans. The common dog’s lifestyle practices, in an environment totally shared with the human species and where the development of spontaneous tumors can be just as common, places the dog in a unique position that can allow for a better understanding of the development and progression of cancer than traditional models of laboratory animals [5].
2. Osteosarcoma
Some of the comparative age-related biological characteristics of OS between humans and animals, especially in dogs, are interesting. In humans, two peaks of occurrence are described. The largest peak occurs in young patients between 10 and 20 years old. The second group consists of osteosarcoma arising in Northern Europeans secondarily to predisposing diseases such as Paget’s disease of bone, or Li-Fraumeni syndrome. In dogs, the age range for diagnosis of osteosarcoma in epidemiological studies is reported to be from 3 months to very old large-breed dogs, with most of the diagnoses regarding adult animals. In humans, osteosarcoma occurs much more frequently in very young and adolescent subjects, with variable secondary frequency peaks in adults over 65 years of age. In humans, the tumor is mainly localized to the long bones such as the distal femur, the proximal tibia, and the humerus [6]. In the canine species, the different forms of OS predominantly affect large-breed dogs, with localization of the tumor in the appendicular skeleton. The etiology of this tumor is still unknown, although several etiopathogenetic hypotheses have been formulated, including the deletion of TP53 and Rb, which can cause osteosarcomatous transformation of osteoblasts [7]. No comparable predisposing genetic factors have currently been recognized as tumor-promoting factors in dogs or other domestic animals. In veterinary medicine, osteosarcomas are essentially classified in three categories on the basis of the primary localization of the tumor: central, parosteal, and periosteal, where diagnostic features are characterized by production of osteoid and tumor bone and embedded malignant osteogenic cells. In canine and feline osteosarcomas, central osteosarcomas have the highest degree of malignancy with a frequent and fast metastasizing process to the lungs. The genetic susceptibility in human OS is associated with heritable cancer syndromes. The most frequent cases of diseased or predisposed bone are represented in veterinary medicine by trauma, orthopedic implants (foreign bodies), specific genetic abnormalities, chemotherapy, and radiotherapy. There are still very few data available on the etiopathogenesis and the biomolecular and genetic characteristics, such as predisposing and causal factors determining canine osteosarcoma.
The frequency of chromotripsis (a mutational process by which up to thousands of chromosomal rearrangements occur grouped into a single event in localized genomic regions confined to one or a few chromosomes) in OS is high, with a frequency value of approximately 77% [8]. This anomaly in OS generates amplification of genes such as CDK4, MDM2, COPS3, RICTOR, and TERT gains, or disruption of driver oncogenes (TP53, NF) [8].
Treatment of OS in both humans and dogs requires a multidisciplinary approach involving the combined action of surgery with preoperative and postoperative chemotherapy using cytotoxic factors (cisplatin, doxorubicin, high-dose methotrexate/ifosfamide) [9]. Therapeutic advances with neoadjuvant and adjuvant chemotherapy have led to improved overall survival rates in OS patients with non-metastatic disease at diagnosis by up to 70%, but for metastatic patients at diagnosis and with relapse survival rates, they are only 20% [9].
3. MiRNA in Canine and Human Osteosarcoma: Comparative Features
Several studies have investigated the role of microRNAs in human OS by means of miRNA expression profiles. Altered miRNA expression and a unique miRNA signature have been identified and associated with the risk of metastasis and a specific response to chemotherapy [10,11,12,13,14]. Since the discovery of miRNAs, few studies have dealt with the association between deregulation of miRNAs and canine OS [15]. Nevertheless, these studies and our own previous studies evaluating miRNA deregulation in naturally occurring canine cancer (such as spontaneous OS), demonstrate that, similarly to its human counterpart, aberrant miRNA expression likely contributes to tumor biology and progression [16,17,18,19].
Thayanithy et al. postulate that multiple microRNAs present at the 14q32 locus in human OS, compared with normal bone tissue, result in downregulation, and that the epigenetics modifications of this locus may contribute to the related alterations. The combinatorial treatment with DNA and chromatin-modifying drugs (such as 5-AzadC) may also activate different miRNAs at the 14q32 locus and significantly modify the lower expression of cell-cycle genes in treated Saos2 OS cell cultures [20]. Sarver A. et al. confirm—on a series of human OS tissue and canine OS tissue and cell lines— 14q32 miRNA downregulation, using miR-382 as a representative of 14q32 miRNAs in human OS and miR-134 and miR-544 in canine OS. In particular, they show for both species an evident association between 14q32 miRNAs decreased expression level and poor outcome in OS patients. Those data suggest that the dysregulation of the 14q32 miRNA cluster may represent a conserved mechanism responsible also for the aggressive and invasive biological behavior of OS in both humans and dogs [21].
MiR-196 is located in the regions of homeobox (HOX) clusters and could be involved in the regulation of those genes (HOXC8, HOXB8, HOXD8, and HOXA7) by playing an important role in cellular development [22]. Its overexpression was assessed in several human cancers, in particular glioblastomas [23], hepatocellular carcinoma [24], and colon cancer [25]. Yang et al. show a downregulation of miR-196 in OS cell lines and postulate that this miR inhibits HOXA9 to promote proliferation and migration of human OS cells [26]. Pazzaglia et al. confirm a downregulation of miR-196a in OS of both species, describing an increase of its target, Annexin 1, in tissues and cell lines. The effects of miR-196a overexpression on tumor cell response may be strictly related to species and cell type. The ectopic expression of miRNA-196a in cell lines seems to influence the significant decrease in cell proliferation and also the increase in apoptotic phenomena, especially in the human cell line 143B of OS. From our study, we identified a transient decrease in some cell motility factors in the human OS 143B cell line and canine OS cell line (DAN), and a more sustained decrease in the other human OS cell line (MG63) [27].
Fenger JM et al.in a series of canine OS cell lines and tissues, identified the miRNA expression using the nanoString system of analysis observing 26 overexpressed miRNAs in canine OS tumor samples compared with normal canine osteoblast cells, and about 44 others miRNAs that insetad were downregulated.
MiR-1, miR-9, miR-10b, miR-29a, miR-122, miR-126, miR-199b, miR-200c, and miR-451, all mature miRNAs that share 100% sequence homology between dogs and humans, were afterwards validated. These miR-9 expression levels were found to be significantly higher in primary canine tumor samples when compared with normal canine osteoblasts [28]. Different studies assessed that miR-9 is downregulated in several human and canine cancers (esophageal, ovarian, colon, renal, gastric, etc.) [29,30,31,32], whereas other investigators observed an overexpression of this miRNA in other forms of cancer such as biliary tract cancer, breast cancer, brain tumor, and lung cancer [33,34,35,36].
Gao et al. show that inhibition of miR-9 decreases the OS cell proliferation by targeting p16; meanwhile, Zhu et al. evidence this role as oncogene-promoting proliferation by targeting Grap2 and cyclin D interacting protein [37,38]
Over the last several years, the role of miR-9-5p in breast cancer, osteosarcoma, and hepatocellular carcinoma has been evaluated, and its overexpression of miR-9-5p has shown correlations with advanced tumor stages and poor prognosis [39,40]. A study about a large series of OS specimens and corresponding non-cancerous bone tissues revealed a miR-9 expression increase, and this high expression is associated with the worst outcome [41].
Among the various miRNAs considered in the etiopathogenetic and biological mechanisms of progression of many tumors, the miR-34 family (miR34a, miR-34b, and miR-34c) has been investigated for a long time, obtaining important results that suggest that members of this family play an important role as tumor suppressors in a wide variety of human spontaneous tumors [43]. It has now been established that these miRNAs can interfere with the mRNA of various cellular proliferative and anti-apoptotic regulatory factors by negatively controlling their expression, which can thus lead to cell cycle arrest, cell senescence, and apoptosis [44]. It is now also clear that p53 trans activates the microRNAs (miRNAs) of the 34 families. Several studies have shown a decrease in miR-34 expression in human OS associated with an increase in several target genes known to be involved in the mechanisms of tumorigenesis, such as MET, SIRT1, and CDK6 [45,46]. Lopez et al. demonstrated that miR-34a expression is significantly reduced in primary canine OSA tissues and cell lines compared with normal canine osteoblasts. Stable overexpression of miR-34a in canine OS cell lines also reduced the expression of angiogenic factors such as VEGFA (Vascular Endothelial Growth Factor) with a concomitant consequent decrease in cell invasion and migration [47]. Furthermore, lower expression of mi-34a in tumor and plasma has also been associated with poor prognosis and chemo resistance, and, recently, a new bioengineered tRNA/miR-34a prodrug demonstrated important antitumor activity in a canine model of OS [4].
Some of our previous data show increased expression of miR-106b and miR.93-5p in human OS, although OS p53wt cells responded to ectopic overexpression of miR-93-5p more significantly, resulting in increased proliferation and tissue invasion compared with cells lacking functional p53. Analysis of the miR-106b cluster (miR-106b, miR-93-5p, and miR-25) in human and canine OS shows variable expression of these molecules. No significant difference with corresponding normal bone was observed in miR-106b and miR-25 expression, while miR-93-5p expression was increased in all OS samples, with higher expression levels in the canine subgroup compared with the human subgroup [48,49] (Figure 1). Zhang et al. show that miR-93-5p, via p21, increases cell proliferation in some forms of nasopharyngeal carcinoma, while inhibiting apoptosis in human hepatocellular carcinoma [50]. In our study, after the downregulation of miR-93-5p in both subgroups, p21 expression was increased at both the mRNA and protein levels and, in particular, the introduction of the inhibitor miR-93-5p caused a cellular response in cultures of human OS 143B and canine OS DAN, which differed in the most intense functional impact in DAN.
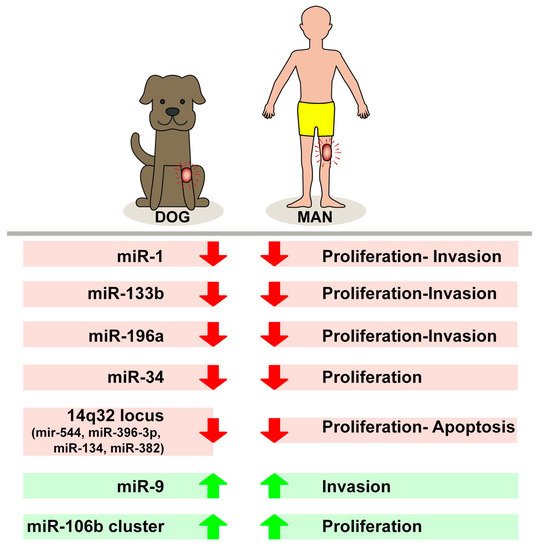
Figure 1. Different levels of expression of tested microRNAs in biological behavior of canine and human osteosarcomas.
This entry is adapted from the peer-reviewed paper 10.3390/cells10020428
 Encyclopedia
Encyclopedia
