Small double-strand RNAs (dsRNA) might target promoter regions of genes thereby activating transcription of targets a process known as RNA activation (RNAa). Small activating RNAs (saRNAs) involved in RNAa have been successfully used to activate gene expression in cultured cells as well as in different in vivo models. Thus, this technique might allow to develop various biotechnological applications without the need to synthesize hazardous construct systems harboring exogenous DNA sequences. The recent success of Covid-19 vaccinations is an excellent example of the therapeutic use of RNAs.
- small interfering RNAs (siRNAs)
- microRNAs (miRNAs)
- small activating RNAs (saRNA)
- molecular mechanism
- neuronal development
- cardiac development
- cancer
- therapeutics
1. Introduction
Small interfering RNAs (siRNAs) and microRNAs (miRNAs), key regulators of gene expression, are recognized as small double-stranded RNA (dsRNA) molecules [1,2,3]. They are loaded onto Ago proteins generating an active Ago–RNA complex, which regulates gene expression at transcription and post-transcription levels [4,5]. Several studies have demonstrated that dsRNAs mostly inhibit gene expression either by chromatin modifications or by translation inhibition [6,7,8,9]. A new kind of small dsRNA, small activating RNAs (saRNAs), are able to induce rather than inhibit gene expression by targeting promoter sequences of some genes, which is termed “RNA activation” (RNAa) [10,11,12,13]. saRNAs are 21 nucleotides in length and act in an Ago2-dependent manner in mammals similar to RNAi [11]. Although the first reports showed that gene expression can be activated by synthetic dsRNAs targeting promoter regions, more recent studies confirmed that RNAa is an evolutionarily conserved mechanism present in diverse eukaryotic organisms ranging from nematodes to humans [14,15]. Like small double-stranded activating RNAs, it has been evidenced that some miRNAs activate gene expression through targeting both promoter sequence and/or AU-rich elements in UTRs [11,16,17]. Several independent studies have shown that a new group of activating RNAs could be generated from natural antisense transcripts (NATs) [14,15,16]. Antisense transcripts, known as long non-coding RNAs, are transcribed from the opposite DNA strand of a protein-coding gene locus, which is complementary to the corresponding coding RNA. They are functional elements, expressed in a tissue-specific manner, and generally low in abundance [17]. Recent reports demonstrate that antisense transcripts regulate their sense (protein-coding) partners through diverse transcriptional and post-transcriptional mechanisms [18,19]. While most antisense transcripts suppress corresponding sense gene expression, they can also be regarded as targets for saRNAs [13,15]. Notably, blocking the interaction of the sense (mRNA) and antisense transcripts (forming a natural duplex) and/or destruction of antisense transcripts by siRNAs or single-stranded oligonucleotides (antagoNATs) results in locus-specific transcriptional de-repression and upregulation of the gene, possibly by activation of the RNAi machinery [14,15,16]. Apart from promoter sequences, which are known as the main target sites for saRNAs, gene activation by small RNA fragments could operate in any genomic region where antisense transcripts are presented. Despite tremendous advantages offered by saRNAs, there are still critical challenges limiting the development of these cutting-edge therapeutics. The most significant challenges include low siRNA stability, degradation and opsonization in the bloodstream, and off-target effects (Table 1). Nanoparticle-mediated delivery systems can open a new way to overcome these problems.
Table 1. Pros and cons of using saRNAs as therapeutics.
|
Advantages |
Disadvantages |
|---|---|
|
Effective gene activation |
Poor cellular uptake |
|
Locus-specific activation of gene transcription, including undruggable targets |
High sensitivity to RNase degradation |
|
Easy to manufacture |
Renal clearance |
|
Cost-effectiveness |
Repeated administration |
|
Low toxicity |
Off-target effects |
|
Easy large-scale production |
Activation of Toll-like receptors |
|
Poor immunogenicity |
2. Small Activating RNAs Are Involved in Locus-Specific Induction of Neural Genes
saRNA-mediated locus-specific gene activation has been studied in neural cells. Based on many studies, two models of locus-specific gene activation have been reported, promoter-targeted duplex RNA activating and natural antisense disruption, leading to changes in chromatin structure [8,14,15,20]. Several studies show that endogenous saRNAs selectively activate gene expression in neurons through targeting promoter sequences [21,22]. Kuwabara et al. have reported a neural-specific dsRNA of about 20 bp containing the NRSE (neuron restrictive silencer element) sequence. This sequence, which is defined as the NRSE/RE1, is localized within promoter regions of neuron-specific genes and is recognized by neuronal restricted silencing factor/RE-1 silencing transcription factor (NRSF/REST) leading to neuron-specific gene suppression in a non-neuronal cell. During an early stage of neurogenesis, the NRSE dsRNA induces the expression of genes containing the NRSE/RE1 sequence in their promoters. Indeed, the noncoding dsRNA, as an endogenous activating RNA, interacts with NRSF/REST machinery and modulates its function. Through this process, neural stem cells can be differentiated into neuronal and glial cells [21]. Cell-mediated brain repair suffers from poor survival rate of transplanted cells and the low efficiency of differentiation into neuronal cells [23]. Diodato and his colleagues have used pre-miRNAs (as activating RNAs) to increase the expression of Emx2, a human homeobox transcription factor modulating a number of developmental mechanisms such as development of cerebral cortex. Their results showed that the transactivation of Emx2 can result in delayed differentiation, self-renewal, and decreased death of neuronally committed precursors [24]. Exogenous saRNA-mediated gene activation in the brain has been reported by Fimiani and his co-workers [22]. Synthesized saRNAs induce the Foxg1 transcription factor, a key regulator of cortico-cerebral development and function. Foxg1 allele duplication and deletion in humans results in West and Rett syndromes, respectively [25]. As a prospective RNAa therapy of Rett syndrome, Foxg1 gene expression in neural cells has been induced in vitro and in vivo by intraventricular injection of saRNA to mouse neonates [22]. NATs destruction is another alternative locus-specific gene activation mechanism, which holds great therapeutic promise. For example, Modarresi et al. have studied the regulatory role of a natural antisense transcript in the brain-derived neurotrophic factor (BDNF) locus [14]. BDNF is a member of the neurotrophin growth factor family and is essential for neuronal maturation, plasticity, memory processes, and differentiation [26,27,28,29,30,31]. Interestingly, locus-specific induction of Bdnf expression is evidenced, both in vitro and in vivo, following degradation of a natural antisense transcript by single-stranded oligonucleotides (term antagoNAT) or siRNAs [14]. Induction of endogenous Bdnf expression following Bdnf-AS repression in neurospheres induced neuronal progenitor cell differentiation. Corresponding to these in vitro experiments, in vivo Bdnf mRNA and protein expression is also induced upon intracerebroventricular injection of Bdnf-antagoNAT9 in mice. It seems that in the case of Bdnf induction, a RNAa mechanism applies through regulating epigenetic modifications, namely reduction of the H3K27me3 repressive mark at the Bdnf locus. Transient induction of neurotrophin expression using the RNAa system is suggested as a pharmacological approach for several neurological disorders, as reduced neurotrophin expression has been observed in different neurodegenerative and neurodevelopmental disorders [14].
Spinal muscular atrophy (SMA) is another example of a neuromuscular disorder [32]. Insufficient expression of functional survival motor neuron protein (SMN), which is correlated with disease severity, leads to muscle weakness after birth [33,34,35]. Several therapeutic efforts have focused on increasing SMN expression [32]. d’Ydewalle et al. have identified a natural antisense transcript in SMN (SMN-AS) locus, which transcriptionally suppresses SMN expression through epigenetic modifications. Importantly, knockdown of the antisense transcript induces SMN transcriptional activity either in patient-derived cells or in the central nervous system of a SMA mouse model in vivo, improving survival of the mice and indicating a novel therapeutic target for SMA [36].
We believe that the next step for clinical translation of RNAa therapeutics for the treatment of various neurodegenerative and neurodevelopmental disorders is the development of novel drug delivery systems.
3. Small Activating RNAs Are Involved in Locus-Specific Induction of Cardiac Genes
RNAa-mediated locus-specific activation of gene transcription has been reported in cardiovascular cells [37,38,39]. In all reported studies, targeting of promoter regions by small dsRNA or small hairpin RNA (shRNA) leads to transcriptional activation of cardiovascular genes, which could open the way for therapeutic strategies. For instance, targeting the promoter region of vascular endothelial growth factor (VEGF) by shRNA results in transcriptional activation, suggesting a therapeutic strategy for myocardial infarction [37]. Targeting the antisense transcript has been recently demonstrated as an alternative RNA activating system in the cyclin-dependent kinase 9 (Cdk9) locus, a key player in cardiac development [15,16]. Cdk9 is associated with specific cyclins to form a heterodimer, Cyclin T/Cdk9, which is also known as the positive transcription elongation factor-b (P-TEFb) [40,41,42]. P-TEFb activates the polymerase II transcription machinery via phosphorylation of the carboxyl-terminal domain (CTD) [43,44]. Therefore, Cdk9 is mainly involved in transcriptional regulation and plays a critical role in several differentiation pathways. Furthermore, Cdk9 regulates cardiac-specific genes including Nkx2.5, Anf, and ß-Myh via interactions with the p300/GATA4 complex, particularly involved in cardiac differentiation [45]. Moreover, we have shown recently that Cdk9 regulates apoptosis in cardiomyocytes by modulating miRNA-1 expression, a critical microRNA for cardiac differentiation [46,47,48]. It is therefore possible that both synthesis and activity of Cdk9 are tightly regulated at the transcriptional and post-transcriptional levels. In this regard, at least three non-coding RNAs are involved in Cdk9 regulation [16]. In the context of normal human cardiomyocytes, Cdk9 activity is suppressed at the protein level via interaction with 7SK non-coding RNA and at the translational level through muscle-specific microRNAs, specifically miR-1 and miR-133 [46,49,50]. We have recently reported a third mode of RNA control in the Cdk9 locus [15]. Small non-coding RNA molecules of 22bp with sequences homologous to the transcript result in transcriptional activation of Cdk9. Interestingly, NATs complementary to the most 3′ and 5′ regions of the gene were identified. Indeed, hybridization of the short single-stranded cognate transcript fragments with antisense transcripts provides the signal for transcriptional activation. The requirement of Argonaute proteins and endogenous antisense transcripts for transcriptional activation indicates that the activating single-stranded small RNAs are processed by the RNAi machinery [51,52]. Similar to siRNA knockdown, antisense transcript distraction following the sense oligoribonucleotide electroporation could represent a secondary phenomenon of the activation of the RNAi machinery. This activation may then result in a change in epigenetic modifications at the locus, leading to the induction of Cdk9 transcription as described for several genes [1,53]. As a functional consequence of RNA activation in the Cdk9 locus, an increased cardiac differentiation potential is observed in ES cells when electroporated with the sense oligoribonucleotide. Interestingly, injection into wild-type blastocysts of RNA-programmed ES cells contributes specifically to heart development in vivo, indicating that a transient RNA activation system is sufficient to create a cardiac differentiation “memory” in cells and may represent a novel tool for RNA–cell reprogramming applied in regenerative medicine.
4. Small Activating RNAs: New Insights into Cancer Therapy
Recently, RNA-based therapeutics have gained more attention in cancer therapy due to their enormous potential to selectively target previously undruggable genes and gene expression modulators. Unlike RNAi, which mostly targets sense transcripts, RNAa, as an alternative and promising new therapeutic strategy, can activate gene expression in a natural manner by targeting antisense transcripts or promoter regions [13,54,55,56]. Tumor suppressor genes, which are mostly suppressed in cancers, could be targeted by saRNA to enhance transcriptional activation and restore a normal cell phenotype.
saRNAs can exert their anticancer effects through induction of cell cycle arrest, cellular senescence, proliferation inhibition, apoptosis induction, metastasis suppression, and multidrug resistance reversal (Figure 1). Although several tumor suppressor genes including E-cadherin, NKX3-1, Wt1, and P53 have been induced by this method, p21 is the most investigated tumor suppressor gene for RNAa-mediated gene activation in several tumors and cell lines [10,57,58,59,60,61,62]. P21 is a negative regulator of the cell cycle and is rarely mutated in cancers, and therefore represents a key target for small RNA activation cancer therapy. In this regard, re-activation of the p21 gene by targeting promoter regions inhibits cell viability and proliferation rates, while it induces apoptotic cell death and sensitizes lung cancer cells to chemotherapeutic agents, providing a new approach in cancer therapy [59,60,63].
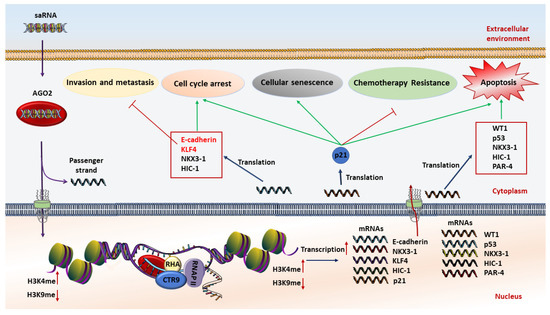
Figure 1. Schematic illustration of the anticancer mechanism of saRNA (small activating RNA)-based therapeutics. At first, saRNAs are loaded on the AGO2 protein. Then AGO2 separates the passenger strand. After that, the complex of saRNA guide strand and AGO2 cross the nuclear and interact with promoter sequences of interested genes to increase transcription by methylation of H3K4 and/or demethylation of H3K9. The expression level of tumor suppressor genes is restored, resulting in induction of apoptosis, chemoresistance reversal, inhibition of invasion and metastasis, cell cycle arrest, and cellular senescence.
This entry is adapted from the peer-reviewed paper 10.3390/cells10030591
