Bimetal CuFe (copper-iron) nanoparticles, which are based on the earth-abundant and inexpensive metals, have generated a great deal of interest in recent years. The fusion of the unique properties of copper and iron in one single entity may result in the formation of nanocomposites with multifunctional properties (magnetic, catalytic, electrical) and their potential applications in the areas of electronics, photonics, and catalysis.
- bimetal nanoparticles
- copper-iron
- magnetic properties
- catalytic properties
- synthesis
- core@shell
- Janus structures
- battery
- photocatalysis
- water treatment
1. Introduction
Bimetallic composite nanoparticles have generated great interest because the combination, at the nanoscale, of different metals can result in new or enhanced physicochemical properties and vast potential applications in the areas of electronics, photonics, catalysis, and biomedicine [1][2][3][4][5]. Properties of obtained particles depend on and can be tailored according to their architectures (e.g., core@shell or multishell structures, hollow structures, heterostructures, alloys), composition, size, and shape [1][6][7][8][9][10].
Copper (Cu) is a 3rd period transition metal and has some interesting physical and chemical properties, such as catalytic activity, high electrical and thermal conductivity, good ductility, malleability, and tensile strength. Due to the catalytic activity of copper nanoparticles, they find a number of applications, including gas-phase reactions, Ullmann reactions, cross-coupling reactions, A3 coupling reactions, azide-alkyne cycloaddition, photocatalysis, and electrocatalysis [11].
An attribute of nanoparticles exhibiting magnetic properties is the ability to selectively attach functional particles and manipulate them using an external magnetic field produced by an electromagnet or permanent magnet [12]. Among them, iron (Fe) is a class of ferromagnetic materials with high magnetic moment density (218 emu/g) and is magnetically soft. Iron nanoparticles in the size range below 20 nm are in the superparamagnetic regime, and their stable dispersions with high magnetic moment are predicted to have wide range applications including data storage, environmental remediation, catalysis, and disease diagnosis and therapy [13]. Nanoscale zero-valence iron (nZVI) is a strong reducing agent. A large specific surface area and reactivity of these nanoparticles increase the efficiency of removing inorganic and organic pollutants as well as heavy metals. However, due to their small size and magnetic properties, they easily aggregate [14][15][16][17]. Between the variety of transition metal macrocycle complex catalysts with different central metal atoms [14][15], iron-based materials have been identified to exhibit the best activity for ORR under various metal loadings [16][17][18][19][20][21][22][23].
On account of their properties, nanomaterials based on copper and iron can effectively replace rare and expensive noble-metal catalysts commonly employed in commercial chemical processes. However, the synthesis and use of both copper and iron nanoparticles is still a challenge due to the high tendency of these materials to oxidize under atmospheric conditions. Therefore, complex nanoparticles (e.g., core@shell, alloys) have been recently adapted to overcome the instability of copper and iron nanoparticles in the presence of oxygen, water, and several chemicals.
Since the fusion of the unique properties of copper and iron in one single entity promises multifunctionality and potential applications, a lot of effort is put into preparing such nanoparticles containing Cu and Fe. This coupling may result in the formation of nanocomposites with an extraordinary catalytic activity and ferromagnetic properties, which would allow for convenient separation of the nanocomposite catalyst from the reaction system in the applied magnetic field (Figure 1).
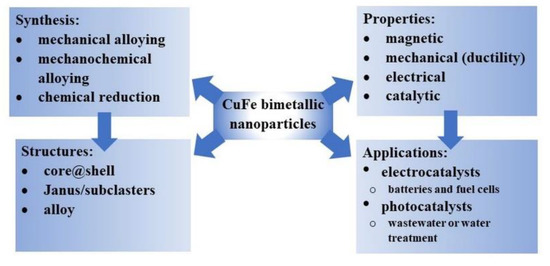
Figure 1. CuFe bimetallic nanoparticles, their synthesis, architecture, properties, and applications.
2. Property of Bimetallic CuFe Nanoparticles
As already mentioned, bimetallic particles composed of two components are expected to display either a combination of the properties associated with each material or new or enhanced properties and capabilities due to coupling between two different materials. The properties of bimetallic nanoparticles can be tuned by varying the concentration of their constituent elements, their architecture, shape, and size. Although bimetallic nanoparticles consist of only two different metals, there are still many types of possible structures: random and ordered alloy, Janus, and core@shell (Figure 2) [5][24].

Figure 2. Types of bimetallic nanoparticles: (a) random alloyed, (b) ordered alloy, (c) sub-clusters/Janus (d) core-shell, (e) multi-shell core-shell, and (f) multiple core materials coated by a single shell material. Yellow and purple spheres represent two different kinds of metal atoms. Reprinted from [5].
The CuFe bimetallic nanoparticles can be oriented in alloy, core@shell, Janus, and other architectures, depending mainly on their synthesis [25][26]. They are known to have face-centered cubic (fcc), body centered cubic (bcc), and hexagonal close-packed (hcp) crystal structures [25][26].
There are a lot of methods for designing various kinds of bimetallic nanoparticles; however, in the case of the CuFe system, both elements are unstable in the presence of air; therefore, using conventional methods is challenging.
There are two general approaches to the synthesis of nanoparticles, i.e., top-down methods and bottom-up methods. Top-down approach involves the breaking down of the bulk material into nanosized structures or particles. Crystal etching, ball milling and grinding are examples of this approach. Bottom-up approach refers to the build-up of a material from the bottom: atom-by-atom, molecule-by-molecule, or cluster-by cluster. Chemical synthesis of nanoparticles is a typical example, but also self-assembly or nanopatterning can be classified as bottom-up techniques. Since Cu and Fe are immiscible in the whole range of composition in equilibrium, a solid solution of the FeCu system can be obtained only by non-equilibrium techniques, such as mechanical alloying [27][28][29] or vapor deposition [30].
CuFe bimetallic nanoparticles were synthesized by mechanical milling [31][32][33], and by chemical reduction [34][35][36][37][38][39][40][41].
3. Applications
As it was shown, the structure depends mainly on the concentration of Cu and Fe in the particles, and mechanical, catalytical, and magnetic properties depend on the structure and composition, and also on the additions such as carbon, graphite, or silicon used for supporting the created nanoparticles. And these properties determinate the applications of bimetallic CuFe nanoparticles. The literature points to applications of CuFe systems in the areas of catalysis and electrochemistry [42][43][44][45], the FeCu nanoparticles are also used as electrocatalysts for energy storage batteries and as photocatalysts and adsorbents for wastewater or water treatment [37][38][41][46][47][48][49][50][51][52][53][54].
Energy storage and conversion are very important for the functioning of modern societies. Energy sources such as batteries and fuel cells are becoming the backbone of a conscious low-carbon economy. Vital for energy conversion, especially in the areas of fuel cells and metal-air batteries, is the oxygen reduction reaction (ORR) [55]. During the ORR process, the O2 molecules are reduced by electrons. They are very difficult to break electrochemically, because the bond O = O has an exceptionally strong bond energy of 498 kJ/mol. The energy barrier can be lowered by electrocatalysts that can activate and cleave bonds. ORR in an aqueous electrolyte occurs mainly by two pathways, one is the direct 4-electron reduction pathway from O2 to H2O, and the other is a 2-electron reduction pathway from O2 to hydrogen peroxide (H2O2). In non-aqueous aprotic solvents and/or in alkaline solutions, the 1-electron reduction pathway from O2 to superoxide (O2−) can also occur [55][56].
Since the introduction of the first fuel cell in 1842, platinum has been the most common catalytic material due to its properties, such as chemical inertness and properties in the field of gas adsorption and dissociation. At the same time, due to platinum’s rarity and costliness, alternative catalytic materials are sought after.
Nam et al. [48] show that FeCu bimetallic nanoparticles are very active and stable electrocatalysts for energy storage batteries. ORR activity for the CuFe alloy with graphitic carbon shells was comparable to that of Pt/C electrocatalysts, CuFe showed kinetic current density much higher than that for single Cu and Fe.
It is known that the presence of toxic metals in water poses a serious threat to living organisms. Examples of the most dangerous toxic metal ions found in wastewater are Hg2+, Cr6+, As3+, Cd2+, Pb2+, Mn2+, Ni2+ [57][58]. They cause renal failure, lung impairment, lung cancer, dermatitis, dyspnea, and damage to the reproductive system, liver, and central nervous system [58][59]. In addition, the excess of the above-mentioned metals adversely affects agriculture and the environment [58][59]. CuFe nanoparticles are also examined as materials for the elimination of heavy metals—such as Cr or As—and metalloids from metallic ores in which Cu prevents the oxidation processes of corrosive metals [36][47].
It would also be worth supplementing the research with the possibility of using CuFe to absorb other toxic metals, such as Pb2+ and Hg2+ or Cd2+.
Bimetallic FeCu nanoparticles can be used to eliminate high concentrations of NO3−-N [49]. These pollutants are produced by agricultural runoff, landfill leachate, leaky septic tanks, municipal storm sewage, animal feed, and industrial waste [60][61] and pose a threat to human health [62].
The textile industry uses dyes that often pollute the water. Dyes are known to be mutagenic, carcinogenic, and toxic to humans and aquatic animals, and therefore adsorbents are needed to remove them [63][64][65]. CuFe and C/CuFe were used as adsorbents to remove indigo blue. Studies have shown that C/CuFe is more effective in removing indigo blue from aqueous solutions than CuFe [35]. CMC-stabilized Fe-Cu bimetal nanoparticles could effectively dechlorinate 1,2,4-trichlorobenzene (1,2,4-TCB) [51]. Cellulose supported Fe-Cu nanoparticles were also applied for reduction of nitroarenes to arylamines [52]. To increase the catalytic activity of the catalysts, porous materials such as mesoporous silica, activated carbon, mesoporous carbon and carbon nanotubes are also used. Compounds such as phenol, benzoic acid, bisphenol A, 2,4,6-trichlorophenol, imidacloprid, ketoprofen, methylene blue, and methyl orange have been effectively removed from water using CuFe nanoparticles dispersed on mesoporous carbon (CuFe-MC) [53].
4. Conclusions
As described in this review, the combination of copper and iron—two well-known and common materials—can provide multi-functional materials for numerous applications. In bulk, copper and iron are slightly soluble in each other, additionally they present a strong tendency to oxidation under atmospheric conditions; therefore, we paid special attention to the synthesis methods of bimetallic CuFe nanoparticles and bimetallic CuFe NPs supported on different materials and the structure of the obtained materials. By mechanical milling, reduction, or two-step reduction, CuFe nanoparticles with alloy, Janus, and core@shell structures were created. Often they were stabilized by carbon, silicon, or cellulose. Structure and properties depend on the size and composition.
Despite the large number of experimental methodologies available, the challenge remains to evolve a simple, efficient, and reliable process for the synthesis of pure CuFe nanoparticles with a defined structure and without any oxide-like impurities. For the challenge of nanoparticle oxidation two techniques seem promising: a combination of a chemical reduction of ferric nitrate and copper nitrate in alcohol solution with annealing carbonization technique, and a high temperature pyrolysis of the copper precursor (chlorophyllin) and the iron precursor (Fe(II) acetylacetonate). While the reduction of copper and iron sulfides with sodium borohydride, and reduction of cupric and ferric nitrates with sodium borohydride in ethylene glycol under Ar seem to be less effective.
CuFe nanoparticles have a great application potential in energy storage, fuel cells, and pollution treatment. CuFe nanoparticles have a great potential for applications in energy storage, fuel cells, and water purification. Therefore, it is important to take advantage of their magnetic and catalytic properties, low cost, and abundance and move them from the laboratory to large-scale applications.
This entry is adapted from the peer-reviewed paper 10.3390/app11051978
References
- Todaka, Y.; McCormick, P.G.; Tsuchiya, K.; Umemoto, M. Synthesis of Fe-Cu Nanoparticles by Mechanochemical Processing Using a Ball Mill. Mater. Trans. 2002, 43, 667–673.
- Sharipova, A.F.; Psakhie, S.G.; Gotman, I.; Gutmanas, E.Y. Smart Nanocomposites Based on Fe–Ag and Fe–Cu Nanopowders for Biodegradable High-Strength Implants with Slow Drug Release. Phys. Mesomech. 2020, 23, 128–134.
- Vishlaghi, M.B.; Ataie, A. Investigation on solid solubility and physical properties of Cu–Fe/CNT nano-composite prepared via mechanical alloying route. Powder Technol. 2014, 268, 102–109.
- Duxin, N.; Brun, N.; Colliex, C.; Pileni, M.P. Synthesis and Magnetic Properties of Elongated Fe−Cu Alloys. Langmuir 1998, 14, 1984–1989.
- Trujillo-Reyes, J.; Sánchez-Mendieta, V.; Colín-Cruz, A.; Morales-Luckie, R.A. Removal of Indigo Blue in Aqueous Solution Using Fe/Cu Nanoparticles and C/Fe–Cu Nanoalloy Composites. Water Air Soil Pollut. 2009, 207, 307–317.
- Feng, H.; Tang, L.; Tang, J.; Zeng, G.; Dong, H.; Deng, Y.; Wang, L.; Liu, Y.; Ren, X.; Zhou, Y. Cu-Doped 2O3 core–shell nanoparticle shifted oxygen reduction pathway for high-efficiency arsenic removal in smelting wastewater. Environ. Sci. Nano 2018, 5, 1595–1607.
- Jiang, D.; Huang, D.; Lai, C.; Xu, P.; Zeng, G.; Wan, J.; Tang, L.; Dong, H.; Huang, B.; Hu, T. Difunctional chitosan-stabilized Fe/Cu bimetallic nanoparticles for removal of hexavalent chromium wastewater. Sci. Total Environ. 2018, 644, 1181–1189.
- Zhu, F.; Ma, S.; Liu, T.; Deng, X. Green synthesis of nano zero-valent iron/Cu by green tea to remove hexavalent chromium from groundwater. J. Clean. Prod. 2018, 174, 184–190.
- Xue, J.; Xiang, H.K.; Ding, H.Q.; Pang, S.L.; Wang, X.H.; Cao, H. Preparation and Magnetic Properties of Carbon Encapsulated Fe-Cu Alloy Nanoparticles. Adv. Mater. Res. 2010, 177, 673–676.
- Carneiro, O.C.; Anderson, P.E.; Rodriguez, N.M.; Baker, R.T.K. Decomposition of CO−H2 over Graphite Nanofiber-Supported Iron and Iron−Copper Catalysts. J. Phys. Chem. B 2004, 108, 13307–13314.
- Xiao, K.; Bao, Z.; Qi, X.; Wang, X.; Zhong, L.; Lin, M.; Fang, K.; Sun, Y. Unsupported CuFe bimetallic nanoparticles for higher alcohol synthesis via syngas. Catal. Commun. 2013, 40, 154–157.
- Han, Z.; Dong, Y.; Dong, S. Copper–iron bimetal modified PAN fiber complexes as novel heterogeneous Fenton catalysts for degradation of organic dye under visible light irradiation. J. Hazard. Mater. 2011, 189, 241–248.
- Lam, F.L.Y.; Hu, X. pH-Insensitive Bimetallic Catalyst for the Abatement of Dye Pollutants by Photo-Fenton Oxidation. Ind. Eng. Chem. Res. 2013, 52, 6639–6646.
- Xia, M.; Long, M.; Yang, Y.; Chen, C.; Cai, W.; Zhou, B. A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution. Appl. Catal. B Environ. 2011, 110, 118–125.
- Lin, H.; Zhong, X.; Ciotonea, C.; Fan, X.; Mao, X.; Li, Y.; Deng, B.; Zhang, H.; Royer, S. Efficient degradation of clofibric acid by electro-enhanced peroxydisulfate activation with Fe-Cu/SBA-15 catalyst. Appl. Catal. B Environ. 2018, 230, 1–10.
- Xiao, K.; Qi, X.; Bao, Z.; Wang, X.; Zhong, L.; Fang, K.; Lin, M.; Sun, Y. CuFe, CuCo and CuNi nanoparticles as catalysts for higher alcohol synthesis from syngas: A comparative study. Catal. Sci. Technol. 2013, 3, 1591–1602.
- Sepúlveda, P.; Rubio, M.A.; Baltazar, S.E.; Rojas-Nunez, J.; Llamazares, J.S.; Garcia, A.G.; Arancibia-Miranda, N. As(V) removal capacity of FeCu bimetallic nanoparticles in aqueous solutions: The influence of Cu content and morphologic changes in bimetallic nanoparticles. J. Colloid Interface Sci. 2018, 524, 177–187.
- Nam, G.; Park, J.; Choi, M.; Oh, P.; Park, S.; Kim, M.G.; Park, N.; Cho, J.; Lee, J.-S. Carbon-Coated Core–Shell Fe–Cu Nanoparticles as Highly Active and Durable Electrocatalysts for a Zn–Air Battery. ACS Nano 2015, 9, 6493–6501.
- Hosseini, S.M.; Ataie-Ashtiani, B.; Kholghi, M. Nitrate reduction by nano-Fe/Cu particles in packed column. Desalination 2011, 276, 214–221.
- Zin, M.T.; Borja, J.; Hinode, H.; Kurniawan, W. Synthesis of Bimetallic Fe/Cu Nanoparticles with Different Copper Loading Ratios. Int. Sch. Sci. Res. Innov. 2013, 7, 1031–1035.
- Cao, J.; Xu, R.; Tang, H.; Tang, S.; Cao, M. Synthesis of monodispersed CMC-stabilized Fe–Cu bimetal nanoparticles for in situ reductive dechlorination of 1, 2, 4-trichlorobenzene. Sci. Total. Environ. 2011, 409, 2336–2341.
- Karami, S.; Zeynizadeh, B.; Shokri, Z. Cellulose supported bimetallic Fe–Cu nanoparticles: A magnetically recoverable nanocatalyst for quick reduction of nitroarenes to amines in water. Cellulose 2018, 25, 3295–3305.
- Wang, Y.; Zhao, H.; Zhao, G. Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2015, 164, 396–406.
- Chun, C.L.; Baer, D.R.; Matson, D.W.; Amonette, J.E.; Penn, R.L. Characterization and Reactivity of Iron Nanoparticles prepared with added Cu, Pd, and Ni. Environ. Sci. Technol. 2010, 44, 5079–5085.
- Ray, A.; Mukhopadhyay, I.; Pati, R.K. (Eds.) Electrocatalysts for Fuel Cells and Hydrogen Evolution—Theory to Design; IntechOpen: London, UK, 2018.
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput. Mater. 2019, 5, 78.
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377.
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418.
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216.
- Hiscock, K.; Lloyd, J.; Lerner, D. Review of natural and artificial denitrification of groundwater. Water Res. 1991, 25, 1099–1111.
- Joekar-Niasar, V.; Ataie-Ashtiani, B. Assessment of nitrate contamination in unsaturated zone of urban areas: The case study of Tehran, Iran. Environ. Earth Sci. 2008, 57, 1785–1798.
- Nolan, B.T.; Ruddy, B.C.; Hitt, K.J.; Helsel, D.R. Risk of Nitrate in Groundwaters of the United StatesA National Perspective. Environ. Sci. Technol. 1997, 31, 2229–2236.
- Ayazi, Z.; Khoshhesab, Z.M.; Amani-Ghadim, A. Synthesis of nickel ferrite nanoparticles as an efficient magnetic sorbent for removal of an azo-dye: Response surface methodology and neural network modelling. Nanochem. Res. 2018, 3, 109–123.
- Mushtaq, M.W.; Kanwal, F.; Imran, M.; Ameen, N.; Batool, M.; Batool, A.; Bashir, S.; Abbas, S.M.; Rehman, A.U.; Riaz, S.; et al. Synthesis of surfactant-coated cobalt ferrite nanoparticles for adsorptive removal of acid blue 45 dye. Mater. Res. Express 2018, 5, 035058.
- Hassani, A.; Çelikdağ, G.; Eghbali, P.; Sevim, M.; Karaca, S.; Metin, Ö. Heterogeneous sono-Fenton-like process using magnetic cobalt ferrite-reduced graphene oxide (CoFe2O4-rGO) nanocomposite for the removal of organic dyes from aqueous solution. Ultrason. Sonochem. 2018, 40, 841–852.
