Skin models offer an in vitro alternative to human trials without their high costs, variability, and ethical issues. Perspiration models, in particular, have gained relevance lately due to the rise of sweat analysis and wearable technology.
- skin models
- skin phantom
1. Introduction
The skin is our first interface with the surrounding environment, simultaneously acting as a protective barrier and a sensing platform. Skin studies are necessary to assess its real nature despite the limitations that experimentation on living tissue present. Researchers have been using non-invasive methods for the past decades, with the use of skin replica methods being a well-known example for the study of surface microtopography [1]. In recent years, there has been an increase in the implementation of in vitro models at multiple levels. This way, ethical issues and the high variability of human tests are avoided, while speeding up the testing process thanks to their longer storage stability and lower costs [2]. We have divided the skin models found in the literature into four main groups, depending on their purpose: physical phantoms, skin substitutes, skin-on-a-chip, and perspiration models (Figure 1).
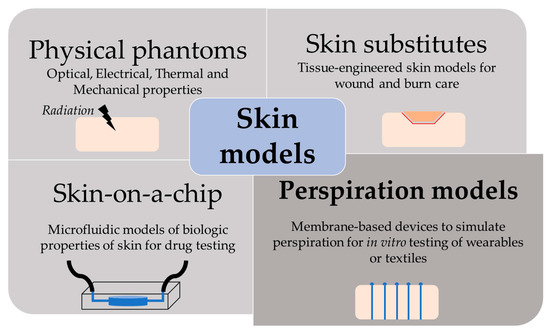
Figure 1. Scheme of the different purpose and applications of skin models.
2. Perspiration Models
The perspiration simulation works found in the literature model the sweat gland as a fluid conduit, discarding to replicate their role on sweat generation. Sweat generation, which takes place in the secretory coil, is replaced by pumping mechanisms, such as syringe pumps and hydrostatic pressure. Although syringe pumps allow for directly setting a specific flow rate and are easier to use, hydrostatic control is sometimes preferred due to its more stable response. Therefore, the models presented are focused on the transport of sweat from a fluid source to a skin-like surface.
Most works use laser systems, specifically, CO2 lasers, to machine the holes that will simulate sweat gland ductal conduits. CO2 lasers have been extensively used in rapid manufacturing due to their high versatility and cost-effective operation and maintenance [43]. Their infrared radiation (wavelength range of 9–12 µm) is capable of removing material by thermal ablation. The features’ characteristics (width, depth, and geometry) can be tuned by controlling laser parameters, such as the power, speed, and focal distance. These benefits have made CO2 lasers a well-known tool in microfluidics laboratories for rapid prototyping, without the need for molds or complex equipment [44]. In particular, they are adequately suited for fabricating passing holes in a polymeric substrate, which is the case of the perspiration models listed. Section 3.2 lists other perspiration models, which may or may not be used as a wearable interface, that use alternative fabrication methods or materials.
2.1. Laser-Machined Membranes
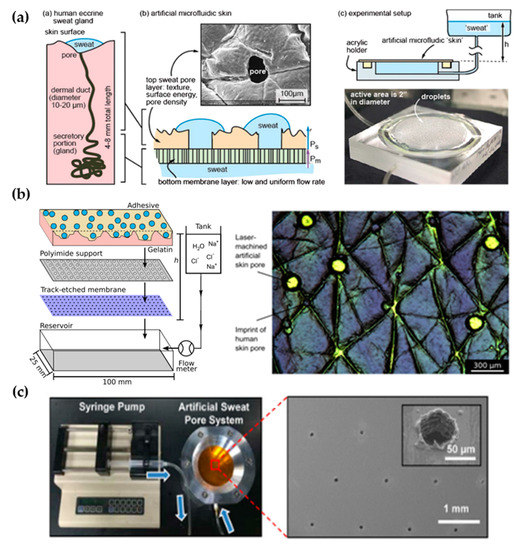
| Work | Sweat Gland Diameter (µm) | Sweat Gland Length (mm) | Sweat Gland Density (cm−2) | Contact Angle (°) | Roughness Ra (µm) | Sweat Rate (µL/min·cm2) | Fabrication Method | Flow Control |
|---|---|---|---|---|---|---|---|---|
| Human skin [32] | 10–20 | 1–4 | 100–550 | 80–110 [41] | 10–50 (RMS) [35] | 0.2–4 | - | - |
| Hou et al. [34] | 80 | <0.1 | 200 | θa= 76 | - | 0.8–5 | CO2 laser | Hydrostatic pressure |
| Eiler et al. [45] | 86.8 ± 17.5 | <0.1 | 100 | 69.2 ± 3.6 | 12.1 ± 1.3 | 0.5–2 | CO2 laser | Syringe pump |
| Hansen et al. [46] | 250 | <0.1 | 100 | 77.5 ± 0.8 | 8.4 ± 4.5 | 0.5–2 | CO2 laser | Hydrostatic pressure |
| Koh et al. [47] | 60 | <0.1 | 100 | - | - | 1.3 | CO2 laser | Syringe pump |
| Liu et al. [48] | 20 ± 3 | <0.1 | 620 | - | - | - | Lift-off + CO2 laser | - |
2.2. Alternative Approaches
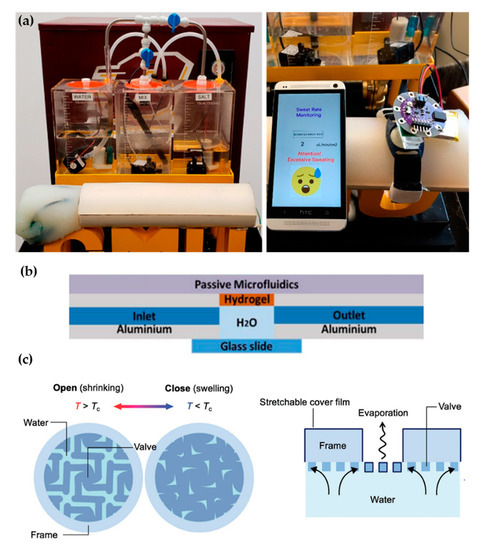
Recent proposals of perspiration models are not reduced solely to laser-machined membranes. With a methodology similar to thermal manikins, Brueck et al. [49] designed an arm mold with casted silicone, which was coupled with a complex electronic system capable to control the desired sweat rate dispensed by a peristaltic pump (from 1 to 500 µL/min) and the salt concentration of the emerging sweat (from 10 to 200 mM) (Figure 4a). Details on the fabrication of the sweat pores are not given in the publication, probably opting for a system of pipes and holes similar to the one used in thermal manikins. Characterization of the flow rates achievable is validated experimentally by gravimetry.
Alternative fabrication methods have also been used for sweat gland fabrication such as the additive manufacturing by 3D printing proposed by Turcin et al. [50]. The authors fabricated a shell of a torso by fused deposition modelling (FDM) to integrate a sweating device manufactured by stereolithography (SLA). SLA, where the polymerization of a resin takes place by ultraviolet radiation, was chosen for its good surface finish of the products and resolution. Although it has been shown that SLA can arrive at the dimensions required for the sweat gland [51], the sweating device has a minimum diameter of 0.55 mm, which is much larger than the actual sweat gland diameter. This structure could be adopted as an alternative way to distribute sweat generation in a thermal manikin, but its application in sweat wearable testing would be limited.
Hydrogels offer the possibility to recreate sweating directly through their micrometric porous structure. Garcia-Cordero et al. [52] used an agarose hydrogel as a skin-like fluidic interface to their microfluidic wearable device (Figure 4b). The hydrogel was integrated into an aluminum chamber where sweat was pumped, and it diffused through the hydrogel. Although hydrogels are a good substitute for a soft tissue such as the skin, their intricate three-dimensional pore structure does not correspond with the sweat gland structure.
Kim et al. [53] also used a hydrogel to fabricate an artificial perspiration membrane. However, their aim was to create a refrigeration system inspired in the sweating mechanism. The thermoresponsive hydrogel used served as a valve to regulate evaporation rate at the interface depending on temperature (Figure 4c). When the temperature is higher than the critical temperature (Tc), the hydrogel shrinks, producing an enlargement in the evaporation area. This way, the evaporation and cooling effect can be regulated as a function of the temperature. This example further illustrates the wide variety of characteristics and purposes of skin models.
This entry is adapted from the peer-reviewed paper 10.3390/membranes11020150
