The gut microbiota (GM) represents a diverse and dynamic population of microorganisms and about 100 trillion symbiotic microbial cells that dwell in the gastrointestinal tract.
- gut dysbiosis
- microbial metabolites
- diet
1. Introduction
The human body hosts trillions of microorganisms, including bacteria, fungi, archaea, and viruses. These symbiotic microorganisms can be beneficial, neutral or detrimental to the host and play regulatory functions in both health and disease. They can be found in the urogenital organs, respiratory tract, skin surface, and gastrointestinal tract (GIT). About 95% of the symbiotic microorganisms of the human microbiome reside in the gut [1]. Intricate ecological colonies of microorganisms dwell in the GIT and are collectively known as the gut microbiota (GM) [2][3]. The GM comprises mainly bacteria, fungi, bacteriophages, archaea, protozoa, and eukaryotic viruses and about 100 trillion microorganisms are harbored in the human GIT [4][5]. Firmicutes, Bacteroides, Proteobacteria and Actinobacteria represent the major bacteria of the gut [6], followed by Bifidobacterium, Clostridium, Eubacterium, Peptococcus, Provetella, etc. [7]. The small intestine consists of various types of bacteria, with content levels ranging from 104 bacteria/mL to 106–107 bacteria/mL at the ileocecal junction, while the large intestine contains most of the non-spore forming bacteria (from 1011–1012 bacteria/g content) [8]. The population of GM is different for each person and is composed of various bacterial species. Furthermore, the composition of the GM is different at different stages of life. In older age individuals, increased Firmicutes and Bacteroidetes populations can be observed as compared to in younger individuals [9]. The GM is involved in metabolic processes and defense mechanisms and represents a dynamic and diverse population which impacts on the health and disease of the host. The GM develops the immune system in the intestinal mucosa and protects the host from carcinogens by releasing short-chain fatty acids (SCFA) [3]. Alterations of the GM community are referred to as dysbiosis [4][10], and these can lead to metabolic disorders. However, recent studies have proposed that this can also affect the central nervous system (CNS) because of the microbiota–gut–brain axis (MGBX) [11][12][13]. The brain regulates the secretory and sensory functions of the gut, and the connection between the gut and brain is interceded by physiological channels such as the autonomic nervous system, neuroendocrine system, neuroimmune pathways, and signaling molecules produced by the GM [14]. However, the actual mechanism and relationship between neural dysfunction and gut dysbiosis is elusive [15]. Emerging evidence suggests that gut dysbiosis can cause neurocognitive disorders such as schizophrenia, depression, bipolar disorder, anxiety, post-traumatic stress disorder, obsessive-compulsive disorder, and dementia, as well as the psychological and behavioral symptoms of dementia (Table 1) [12]. Additionally, metabolic syndromes and gut dysbiosis also contribute to Alzheimer’s disease (AD) and effect memory, learning, and hippocampal plasticity [16]. Diet, probiotics, and other therapeutic strategies have positive effects on GM modulation that may be helpful in the treatment of AD, as these factors alter the composition of the GM and have a positive impact on the host, improving the health status of the gut and body overall [3].
Table 1. Changes in microbiome occur during several mental conditions and their related findings.
| Condition | Study | Change in Microbiome | Findings | Reference |
|---|---|---|---|---|
| Depression | Human (n = 90) |
↑Phylum Bacteroidetes, classes Gammaproteobacteria and Bacteroidia, order Bacteroidales, and genera Flavonifractor and Sellimonas. ↓Phylum Firmicutes, class Clostridia, order Clostridiales, family Ruminococcaceae (Subdoligranulum, Faecalibacterium, Ruminococcus 1, and (Eubacterium) coprostanoligenes), and family Christensenellaceae (Christensenellaceae R7 group). |
Low levels of SCFA, anti-inflammatory, and butyrate-producing bacteria may link the GM and the low-grade and chronic inflammation. | [17] |
| Human (n = 16) |
Depression showed a positive correlation with Paraprevotella, negative correlations with Clostridiales, Clostridia, Firmicutes, and the RF32 order. | Intestinal inflammation and integrity markers found to be related to the response to treatment in patients with symptom severity and major depressive disorder. | [18] | |
| Human (n = 111) |
Clostridiales order, Ruminococcaceae family (Clostridium symbiosum and Coprococcus catus) variably differentiated depression severity strata. | Coprococcus catus specifically found to be a contributor to psychiatric functioning. | [19] | |
| Schizophrenia | Human (n = 26) |
↑Proteobacteria, Chaetomium ↓Faecalibacterium, Lachnospiraceae, and Trichoderma. |
Faecalibacterium and Genera Lachnospiraceae allow opportunistic pathogens such as Protobacteria to translocate and are involved in CD4 cell differentiation and can increase gut TH17 cells that can penetrate through the BBB and induce abnormal behavior. | [20] |
| Animal | ↑Lactobacillus and Bifidobacterium. ↓Akkermansia |
Administration of Inulin modulated GM decreased 5-HT and inflammatory cytokines and enhanced BDNF though the MGBX and ameliorated schizophrenia. | [21] | |
| Bipolar disorder | Human (n = 36) |
↓Bifidobacteria to Enterobacteriaceae ratio ↑Enterobacter spp, Faecalibacterium prausnitzii, Clostridium Cluster IV, Atopobium Cluster, and Bacteroides-Prevotella group. |
Brain-gut coefficient of balance (B-GCB) was used as a new concept. | [22] |
| Human (n = 32) |
↑Actinobacteria Coriobacteriia ↓Faecalibacterium and Ruminococcaceae. |
Actinobacteria and Coriobacteriia take part in lipid metabolism correlating with cholesterol levels, found in bipolar patients. | [23] | |
| Anxiety | Human (n = 9) |
↓Microbial richness and diversity. ↑Escherichia-Shigella, Fusobacterium, and Ruminococcus gnavus. |
Enhanced gut permeability due to decrease in SCFA producing bacteria. Increase in the abundance of Escherichia-Shigella, Ruminococcus gnavus, and Fusobacterium bacteria may support systemic inflammation and degrade mucins. | [24] |
| Post-traumatic stress disorder | Animal | Alteration in Bacteroidetes, Firmicutes, Proteobacteria, and Cyanobacteria levels. | Changes in levels of neurotransmitters such as 5-HT, dopamine, and norepinephrine were observed in stressed rats. | [25] |
| Human (n = 93) |
↑Escherichia/Shigella and Enterococcus ↓Autochthonous taxa, Lachnospiraceaeae and Ruminococcaceae. |
Escherichia/Shigella and Enterococcus linked with poor cognition, and higher levels of lipopolysaccharides are linked with neuroinflammation through MGBX. | [26] | |
| Obsessive-compulsive disorder | Human (n = 43) |
↓species richness/evenness (Inverse Simpson, α-diversity), relative abundance of butyrate producing genera (Anaerostipes, Odoribacter, and Oscillospira). | Mean C-reactive protein, but not IL-6 and TNF-α, was increased in the patients. C-reactive protein exhibited mild to strong linkage with psychiatric symptomatology. | [27] |
| Dementia | Human (n = 128) |
↑Enterotype I and III bacteria were associated with dementia. | Serum triglycerides, serum C-reactive protein, and markers of insulin resistance were found in subjects. Fecal lactic acid and ammonia were linked to dementia. | [28][29] |
| Human (n = 77) |
↓Clostridia and its phylum Firmicutes and the Ruminococcus, Ruminococcaceae, Clostridiales at order, family and genus levels. | Decrease in SCFA producing bacteria and indole-3-pyruvic acid was recognized as a signature for prediction and discrimination of AD. | [30] |
↑: Higher, ↓: Lower, AD: Alzheimer’s disease, BDNF: brain-derived neurotrophic factor, GM: gut microbiota, BBB: blood–brain barrier, MGBX: microbiota–gut–brain axis, 5-HT: 5-hydroxytryptamine, SCFA: short-chain fatty acids, IL: interleukin, TNF-α: tumor necrosis factor-α. B-GCB: the ratio of (oxygenated hemoglobin)/(Bifidobacteria to Enterobacteriaceae ratio) to analyze the relationship between brain function and GM. n: number of total subjects in the study but the columns of GM and findings are only showing the data of diseased ones.
2. Impact of GM and Their Metabolites on the Brain
During metabolic processes, the GM can produce several bioactive metabolites that can enter into the bloodstream via absorption into enterohepatic circulation [4]. Metabolites linked to the phenotype of a disease can be recognized by nuclear magnetic resonance (NMR) and mass spectrometry-based metabolomics of body fluids such as urine, feces, or plasma. This makes it possible to carry out joint analyses of the host phenotypes, metabolome, and microbiome to identify mechanistic links [31]. The GM metabolizes a plethora of neurotransmitters and neuromodulators (such as short-chain fatty acids (SCFA), gamma-aminobutyric acid, acetylcholine, dopamine, glutamate, and serotonin) [32][33][34]. Microbial species such as Saccharomyces, Bacillus, Lactobacillus, Escherichia, and Bifidobacterium are known to produce these types of neurotransmitters [33]. Preliminary human studies have revealed that bacterial-based interventions can also change neurotransmitter levels involved in synaptic plasticity (including brain-derived neurotrophic factor), and regulate the activity of N-methyl-d-aspartate and serotonin receptors [34]. Impairment of the GM composition or their metabolites modulates the gut–brain axis [33] and regulates cognition, memory, mood, and social behavior [35][36]. Moreover, dysbiosis may result in the formation of toxic misfolded proteins with a β-sheet conformation that promotes loss of synaptic connections, cellular cell dysfunction, and neurodegeneration [37]. The pathways involved in MGBX are illustrated in Figure 1. Moreover, some of the major microbial metabolites (neurotransmitters) and their role in brain health are exhibited in Table 2.
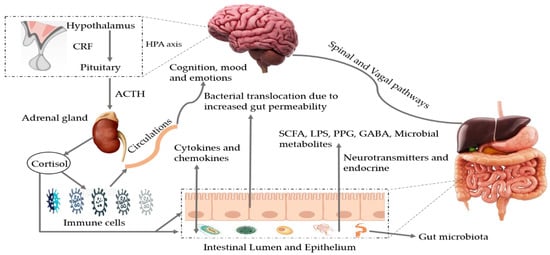
Figure 1. Bidirectional pathways involved in the communication between the gut microbiota and the brain (MGBX). They communicate through vagal and spinal nerves. SCFA, LPS, PPG, GABA, microbial metabolites, other neurotransmitters and endocrine cells are also involved. Dysbiosis can be caused by stress that may alter tryptophan levels, SCFA levels, the immune system, and gut permeability. Additionally, release of cytokines and chemokines (IL-6, IL-1β, IL-8) can lead to neuroinflammation and activation of HPA axis. SCFA: short-chain fatty acids, LPS: lipopolysaccharides, PPG: peptidoglycans, GABA: gama-aminobutyric acid, HPA axis: hypothalamic–pituitary–adrenal axis, CRF: corticotropin-releasing factor, ACTH: adrenocorticotropic hormone.
Table 2. Neurotransmitters produced by gut microbiota and their role in brain function.
| Gut Microbiota | Metabolites | Study | Association in Brain Function | References |
|---|---|---|---|---|
| Lactobacillus, Bifidobacterium | GABA | Human/animal metagenomic | Main inhibitory neurotransmitter of the CNS and a potential mediator between bacterial cells and the host. Regulates depression, anxiety, behavioral and cognitive functions. | [38][39] |
| Akkermansia muciniphila, Lactobacillus plantarum DR7 | Serotonin or 5-HT | Animal metagenomic | Regulates mood, learning, cognition, and memory. | [40][41] |
| Bifidobacterium longum, Clostridium symbiosum, Faecalibacterium prausnitzii, Lactobacillus fermentum | SCFA | Human/animal metagenomic | Regulate neuro-immunoendocrine function, reduce inflammation, promote the synthesis and secretion of neurotransmitters, hormones and suppress permeability of the blood–brain barrier. | [42][43][44] |
| Lactobacillus, Escherichia, Streptococcus, Lactococcus, Bacillus | Dopamine | Human/animal metagenomic | Protects neuron loss, improves motor deficits, cognition, reduced stress and anxiety. | [45][46][47] |
| Escherichia coli, Morganella morganii, Lactobacillus vaginalis, Enterobacter aerogenes | Histamine | Human/animal metagenomic | Regulates depression-like behaviors and impaired sleep-wake cycle. | [48][49][50] |
| Coryneform, Bacteroides vulgatus, Campylobacter jejuni, Lactobacillus | Glutamate | Human/animal metagenomic | Play role in molecular mechanism of learning, memory, and synaptic plasticity. | [51] |
| Lactobacillus, Bacillus | Acetylcholine | Animal metagenomic | Memory, emotional personality, self-care ability, cognition, and social life ability. | [52][53] |
GABA: gamma-aminobutyric acid, CNS: central nervous system, SCFA: short-chain fatty acids, 5-HT: 5-hydroxytryptamine.
This entry is adapted from the peer-reviewed paper 10.3390/nu13020690
References
- De JR De-Paula, V.; Forlenza, A.S.; Forlenza, O.V. Relevance of gutmicrobiota in cognition, behaviour and Alzheimer’s disease. Pharmacol. Res. 2018, 136, 29–34.
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216.
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 118627.
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2020, 19, 55–71.
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904.
- Dahiya, D.K.; Puniya, M.; Shandilya, U.K.; Dhewa, T.; Kumar, N.; Kumar, S.; Puniya, A.K.; Shukla, P. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: A review. Front. Microbiol. 2017, 8, 563.
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533.
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787.
- Doifode, T.; Giridharan, V.V.; Generoso, J.S.; Bhatti, G.; Collodel, A.; Schulz, P.E.; Forlenza, O.V.; Barichello, T. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol. Res. 2021, 105314.
- Madan, S.; Mehra, M.R. Gut dysbiosis and heart failure: Navigating the universe within. Eur. J. Heart Fail. 2020, 22, 629–637.
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.-L. Gut microbiota and dysbiosis in Alzheimer’s disease: Implications for pathogenesis and treatment. Mol. Neurobiol. 2020, 57, 5026–5043.
- Halverson, T.; Alagiakrishnan, K. Gut microbes in neurocognitive and mental health disorders. Ann. Med. 2020, 52, 423–443.
- Shabbir, U.; Rubab, M.; Tyagi, A.; Oh, D.-H. Curcumin and Its Derivatives as Theranostic Agents in Alzheimer’s Disease: The Implication of Nanotechnology. Int. J. Mol. Sci. 2020, 22, 196.
- Montiel-Castro, A.J.; González-Cervantes, R.M.; Bravo-Ruiseco, G.; Pacheco-López, G. The microbiota-gut-brain axis: Neurobehavioral correlates, health and sociality. Front. Integr. Neurosci. 2013, 7, 70.
- Łuc, M.; Misiak, B.; Pawłowski, M.; Stańczykiewicz, B.; Zabłocka, A.; Szcześniak, D.; Pałęga, A.; Rymaszewska, J. Gut microbiota in dementia. Critical review of novel findings and their potential application. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 104, 110039.
- Spinelli, M.; Fusco, S.; Grassi, C. Brain insulin resistance and hippocampal plasticity: Mechanisms and biomarkers of cognitive decline. Front. Neurosci. 2019, 13, 788.
- Liu, R.T.; Rowan-Nash, A.D.; Sheehan, A.E.; Walsh, R.F.; Sanzari, C.M.; Korry, B.J.; Belenky, P. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav. Immun. 2020, 88, 308–324.
- Liśkiewicz, P.; Kaczmarczyk, M.; Misiak, B.; Wroński, M.; Bąba-Kubiś, A.; Skonieczna-Żydecka, K.; Marlicz, W.; Bieńkowski, P.; Misera, A.; Pełka-Wysiecka, J.; et al. Analysis of gut microbiota and intestinal integrity markers of inpatients with major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110076.
- Madan, A.; Thompson, D.; Fowler, J.C.; Ajami, N.; Salas, R.; Frueh, B.; Bradshaw, M.; Weinstein, B.; Oldham, J.; Petrosino, J. The gut microbiota is associated with psychiatric symptom severity and treatment outcome among individuals with serious mental illness. J. Affect. Disord. 2020, 264, 98–106.
- Zhang, X.; Pan, L.-Y.; Zhang, Z.; Zhou, Y.-y.; Jiang, H.-Y.; Ruan, B. Analysis of gut mycobiota in first-episode, drug-naïve Chinese patients with schizophrenia: A pilot study. Behav. Brain Res. 2020, 379, 112374.
- Guo, L.; Xiao, P.; Zhang, X.; Yang, Y.; Yang, M.; Wang, T.; Lu, H.; Tian, H.; Wang, H.; Liu, J. Inulin ameliorates schizophrenia via modulating gut microbiota and anti-inflammation in mice. Food Funct. 2021.
- Lu, Q.; Lai, J.; Lu, H.-F.; Ng, C.; Huang, T.; Zhang, H.; Jiang, J.; Hu, J.; Lu, J.; Lu, S.; et al. Gut microbiota in bipolar depression and its relationship to brain function: An advanced exploration. Front. Psychiatry 2019, 10, 784.
- Painold, A.; Mörkl, S.; Kashofer, K.; Halwachs, B.; Dalkner, N.; Bengesser, S.; Birner, A.; Fellendorf, F.; Platzer, M.; Queissner, R.; et al. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019, 21, 40–49.
- Jiang, H.Y.; Zhang, X.; Yu, Z.H.; Zhang, Z.; Deng, M.; Zhao, J.H.; Ruan, B. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 2018, 104, 130–136.
- Zhou, Q.; Sun, T.; Wu, F.; Li, F.; Liu, Y.; Li, W.; Dai, N.; Tan, L.; Li, T.; Song, Y. Correlation of gut microbiota and neurotransmitters in a rat model of post-traumatic stress disorder. J. Tradit. Chin. Med. Sci. 2020, 7, 375–385.
- Bajaj, J.S.; Sikaroodi, M.; Fagan, A.; Heuman, D.; Gilles, H.; Gavis, E.A.; Fuchs, M.; Gonzalez-Maeso, J.; Nizam, S.; Gillevet, P.M. Posttraumatic stress disorder is associated with altered gut microbiota that modulates cognitive performance in veterans with cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G661–G669.
- Turna, J.; Grosman Kaplan, K.; Anglin, R.; Patterson, B.; Soreni, N.; Bercik, P.; Surette, M.G.; Van Ameringen, M. The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age-and sex-matched controls: A pilot study. Acta Psychiatr. Scand. 2020, 142, 337–347.
- Saji, N.; Murotani, K.; Hisada, T.; Kunihiro, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Niida, S.; Toba, K.; Sakurai, T. Relationship between dementia and gut microbiome-associated metabolites: A cross-sectional study in Japan. Sci. Rep. 2020, 10, 8088.
- Saji, N.; Niida, S.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Toba, K.; Sakurai, T. Analysis of the relationship between the gut microbiome and dementia: A cross-sectional study conducted in Japan. Sci. Rep. 2019, 9, 1008.
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host-Microbe Interplay. Nutrients 2021, 13, 228.
- Pedersen, H.K.; Forslund, S.K.; Gudmundsdottir, V.; Petersen, A.Ø.; Hildebrand, F.; Hyötyläinen, T.; Nielsen, T.; Hansen, T.; Bork, P.; Ehrlich, S.D.; et al. A computational framework to integrate high-throughput ‘-omics’ datasets for the identification of potential mechanistic links. Nat. Protoc. 2018, 13, 2781–2800.
- Fox, M.; Knorr, D.A.; Haptonstall, K.M. Alzheimer’s disease and symbiotic microbiota: An evolutionary medicine perspective. Ann. N. Y. Acad. Sci. 2019, 1449, 3–24.
- Conte, C.; Sichetti, M.; Traina, G. Gut–Brain Axis: Focus on Neurodegeneration and Mast Cells. Appl. Sci. 2020, 10, 1828.
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133.
- Chalazonitis, A.; Rao, M. Enteric nervous system manifestations of neurodegenerative disease. Brain Res. 2018, 1693, 207–213.
- Yang, N.J.; Chiu, I.M. Bacterial signaling to the nervous system through toxins and metabolites. J. Mol. Biol. 2017, 429, 587–605.
- Quigley, E.M. Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94.
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci. Rep. 2020, 10, 14112.
- Yunes, R.; Poluektova, E.; Vasileva, E.; Odorskaya, M.; Marsova, M.; Kovalev, G.; Danilenko, V. A multi-strain potential probiotic formulation of GABA-producing Lactobacillus plantarum 90sk and bifidobacterium adolescentis 150 with antidepressant effects. Probiotics Antimicrob. Proteins 2020, 12, 973–979.
- Zaydi, A.; Lew, L.-C.; Hor, Y.-Y.; Jaafar, M.; Chuah, L.-O.; Yap, K.-P.; Azlan, A.; Azzam, G.; Liong, M.-T. Lactobacillus plantarum DR7 improved brain health in aging rats via the serotonin, inflammatory and apoptosis pathways. Benef. Microbes 2020, 11, 753–766.
- Yaghoubfar, R.; Behrouzi, A.; Ashrafian, F.; Shahryari, A.; Moradi, H.R.; Choopani, S.; Hadifar, S.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; et al. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci. Rep. 2020, 10, 22119.
- Lee, J.; d’Aigle, J.; Atadja, L.; Quaicoe, V.; Honarpisheh, P.; Ganesh, B.P.; Hassan, A.; Graf, J.; Petrosino, J.F.; Putluri, N.; et al. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Post-Stroke Recovery in Aged Mice. Circ. Res. 2020, 127, 453–465.
- Xu, R.; Tan, C.; He, Y.; Wu, Q.; Wang, H.; Yin, J. Dysbiosis of Gut Microbiota and Short-Chain Fatty Acids in Encephalitis: A Chinese Pilot Study. Front. Immunol. 2020, 11, 1994.
- Blaak, E.; Canfora, E.; Theis, S.; Frost, G.; Groen, A.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455.
- Liu, G.; Chong, H.X.; Chung, F.Y.L.; Li, Y.; Liong, M.T. Lactobacillus plantarum DR7 Modulated Bowel Movement and Gut Microbiota Associated with Dopamine and Serotonin Pathways in Stressed Adults. Int. J. Mol. Sci. 2020, 21, 4608.
- Koutzoumis, D.N.; Vergara, M.; Pino, J.; Buddendorff, J.; Khoshbouei, H.; Mandel, R.J.; Torres, G.E. Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson’s disease. Exp. Neurol. 2020, 325, 113159.
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 195–219.
- Barcik, W.; Pugin, B.; Westermann, P.; Perez, N.R.; Ferstl, R.; Wawrzyniak, M.; Smolinska, S.; Jutel, M.; Hessel, E.M.; Michalovich, D.; et al. Histamine-secreting microbes are increased in the gut of adult asthma patients. J. Allergy Clin. Immunol. 2016, 138, 1491–1494.e7.
- Blasco, M.P.; Chauhan, A.; Honarpisheh, P.; Ahnstedt, H.; d’Aigle, J.; Ganesan, A.; Ayyaswamy, S.; Blixt, F.; Venable, S.; Major, A.; et al. Age-dependent involvement of gut mast cells and histamine in post-stroke inflammation. J. Neuroinflamm. 2020, 17, 160.
- Yamada, Y.; Yoshikawa, T.; Naganuma, F.; Kikkawa, T.; Osumi, N.; Yanai, K. Chronic brain histamine depletion in adult mice induced depression-like behaviours and impaired sleep-wake cycle. Neuropharmacology 2020, 175, 108179.
- Chang, C.-H.; Lin, C.-H.; Lane, H.-Y. d-glutamate and Gut Microbiota in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2676.
- Baxter, M.G.; Crimins, J.L. Acetylcholine Receptor Stimulation for Cognitive Enhancement: Better the Devil You Know? Neuron 2018, 98, 1064–1066.
- Chen, D.; Yang, X.; Yang, J.; Lai, G.; Yong, T.; Tang, X.; Shuai, O.; Zhou, G.; Xie, Y.; Wu, Q. Prebiotic effect of fructooligosaccharides from Morinda officinalis on Alzheimer’s disease in rodent models by targeting the microbiota-gut-brain axis. Front. Aging Neurosci. 2017, 9, 403.
