Postbiotics are health-promoting microbial metabolites delivered as a functional food or a food supplement. They either directly influence signaling pathways of the body or indirectly manipulate metabolism and the composition of intestinal microflora. Cancer is the second leading cause of death worldwide and even though the prognosis of patients is improving, it is still poor in the substantial part of the cases. The preventable nature of cancer and the importance of a complex multi-level approach in anticancer therapy motivate the search for novel avenues of establishing the anticancer environment in the human body. This article summarizes the principal findings demonstrating the usefulness of both natural and synthetic sources of postbotics in the prevention and therapy of cancer. Specifically, the effects of crude cell-free supernatants, the short-chain fatty acid butyrate, lactic acid, hydrogen sulfide, and β-glucans are described. Contradictory roles of postbiotics in healthy and tumor tissues are highlighted. In conclusion, the application of postbiotics is an efficient complementary strategy to combat cancer.
- microbiome
- colorectal cancer
- intestinal metabolome
- GPR81
- SCFA
- functional food
Introduction
-
Prebiotics are non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of a limited number of bacteria in the colon, thus improving the host’s health.
-
Probiotics are living microorganisms that, when administered in adequate amounts, confer a health benefit on the host.
-
Synbiotics are combinations of prebiotics and probiotics that have beneficial effects on the gut microbiome.
-
Postbiotics are substances released by or produced through the metabolic activity of the microorganisms which directly exert beneficial effects on the host.
While the research on probiotics has begun more than 100 years ago, postbiotics are a newcomer in the field of science, even though the beneficial effects of fermented food have been known for millennia. Until recently, the studies have been focused on the effects of probiotics, mainly lactic acid-producing bacteria such as Lactobacillus or Bifidobacterium, and on prebiotics such as dietary fiber, human milk oligosaccharides, lactulose, and inulin derivatives. However, the increasing body of evidence suggests that the overall metabolism of the intestinal microflora is more important than the presence or absence of any particular microbial species. The postbiotics research was initiated by studies employing the cell-free supernatants (CFSs) from bacterial fermentation followed by primary microbial metabolites such as lactic acid and short-chain fatty acids (SCFA). However, there are numerous other interesting molecules including the odorous gas hydrogen sulfide (H2S).
Metabolic signaling in cancer
Here, we hypothesize that an important component of the anti-cancer effects of postbiotics is the induction of cell response to metabolic and oxidative stress. As microbial metabolic waste products, postbiotics disturb the metabolic homeostasis of host cells, causing metabolic and oxidative stress. Lactate has been shown to stimulate mitochondrial production of reactive oxygen species (ROS) by increasing the NADH/NAD+ ratio and serving as a substrate of lactate oxidase. Butyrate has also been shown to stimulate ROS levels, although the exact mechanism is unknown. H2S covalently modifies sulfhydryl moieties of proteins and inhibits mitochondrial cytochrome c oxidase enhancing the ROS release.
The adaptive response of the cells is driven by increased activity of stress signaling pathways, including, but not limited to, the energy sensor AMP-activated protein kinase (AMPK), the family of sirtuin deacetylases (SIRT1-7), the metabolic transcription coactivator PGC1α, the antioxidant transcription factor NFE2L2 (Nrf2), and the stimulation of specific survival processes including mitochondrial biogenesis and autophagy. The enhanced stress signaling protects normal tissues against carcinogenesis by suppressing cell proliferation, mutagenesis, and tissue inflammation. Specifically, AMPK activity mimics the cancer-preventing effects of caloric restriction by enhancing autophagy and suppressing mTOR signaling. It is also an activator of sirtuins and PGC1α that inhibit the cell cycle and nuclear factor kappa B (NFκB) signaling. In addition, Nrf2-driven transcription stimulates the antioxidant, detoxification, and DNA repair capacity of the cells. It is important to note that butyrate induces Nrf2 directly through its effect on histone acetylation.
On the other hand, the increased production of mitochondrial ROS accompanied by the enhanced stress signaling frequently occurs in cancer cells as a byproduct of metabolic transformation. Moreover, it is indispensable for the progression of the established malignant tumors due to its pro-survival effect. However, such deregulated signaling may be overwhelmed by external metabolic stress, including postbiotics. Therefore, postbiotics also suppress the progression of established tumors by exaggerating the metabolic and oxidative stress, causing programmed cell death (Figure 1).
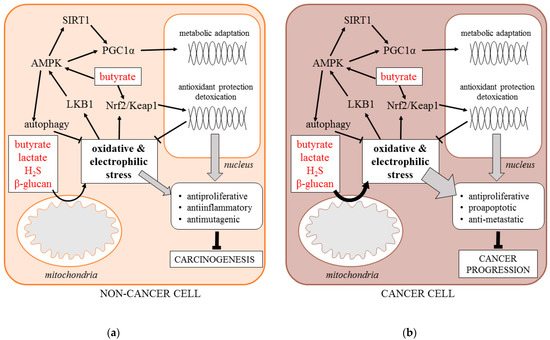
Figure 1. Signaling response of non-cancer (a) and cancer cell (b) to postbiotics (AMPK—5’ AMP-activated protein kinase, H2S—hydrogen sulfide, Keap1—Kelch Like ECH Associated Protein 1, LBK1—liver kinase B1, Nrf-2—Nuclear factor erythroid 2-related factor 2, PGC1α—Peroxisome proliferator-activated receptor gamma coactivator 1-alpha, SIRT1—Sirtuin 1).
For more info see: [1], [2], [3]
This entry is adapted from the peer-reviewed paper 10.3390/molecules26061528
References
- Jaroslav Zelenka; Martina Koncošová; Tomáš Ruml; Targeting of stress response pathways in the prevention and treatment of cancer. Biotechnology Advances 2018, 36, 583-602, 10.1016/j.biotechadv.2018.01.007.
- Jaroslav Zelenka; Ales Dvorak; Lukáš Alán; L-Lactate Protects Skin Fibroblasts against Aging-Associated Mitochondrial DysfunctionviaMitohormesis. Oxidative Medicine and Cellular Longevity 2015, 2015, 1-14, 10.1155/2015/351698.
- Nikola Vrzáčková; Tomáš Ruml; Jaroslav Zelenka; Postbiotics, Metabolic Signaling, and Cancer. Molecules 2021, 26, 1528, 10.3390/molecules26061528.
