Diabetes mellitus has become a serious and chronic metabolic disorder that results from a complex interaction of genetic and environmental factors, principally characterized by hyperglycemia, polyuria, and polyphagia.
- diabetes mellitus
- epidemiology
- biochemistry
- metabolic disorder
- diagnosis
- complications
- management of diabetes
1. Introduction
Diabetes mellitus (DM) is primarily characterized by high blood glucose levels (hyperglycemia), polydipsia, and polyphagia. DM is one of the most common metabolic disorders that is increasing at an alarming rate all over the world [1,2,3]. There are mainly four common types of DM. Type 1 DM (T1DM) is caused by the autoimmune annihilation of the pancreatic-β cell with no insulin production [4]. This type is also called insulin-dependent diabetes mellitus (IDDM) [5,6]. This type of DM is seen in childhood and includes 5–10% of total diabetes patients [1]. The major type of diabetes is Type 2 DM (T2DM), which is caused due to insufficient production of insulin or desensitization of insulin receptors that precludes the entry of glucose into the cell [7,8]. There is another type of diabetes called gestational diabetes mellitus (GDM) that occurs only during pregnancy. Monogenic diabetes, which is often misdiagnosed as T1DM or T2DM is caused by a mutation in a single gene or a cluster of genes [15,16].
2. Epidemiology of Diabetes
The disease burden related to diabetes is high and rising in every country, fueled by the global rise in the prevalence of obesity and unhealthy lifestyles. The latest estimates show a prevalence rate of 11.1% with diabetes in 2019, expected to rise to 13% by 2045 in Northern America and Caribbean regions [37]. The prevalence rate is most pronounced in the Middle East and North African regions which are predicted to rise 13.9% by 2045 (Figure 1). The lowest prevalence rate (4.7%) is observed in Africa which is expected to increase to 5.2% in 2045 [37]. In general, countries in South-East Asia and South America have either high or intermediate incidences [37]. According to a recent study by Saeedi et al. in 2019 [37], a global prevalence of 463 million people with diabetes was recorded which represents a 9.3% prevalence rate worldwide. By 2030 this prevalence rate is estimated to reach 10.2% and by 2045 to 10.9%. The region-stratified diabetes prevalence is calculated for several countries which display the countries with the highest number of diabetic patients in 2019, worldwide [38]. The top 10 countries or territories for the number of people with diabetes are identified (Figure 2). Among these countries, China has the highest proportion of diabetic patients at around 116 million. India seconds the list at 77 million followed by the United States of America at 31 million demonstrating the US as one of the most diabetic risk countries in the upcoming decade. Pakistan, Brazil, and Mexico are those projected to the high-end diabetic groups with diabetic patients at around 19 million, 16 million, and 12 million, respectively. While Bangladesh falls in the lower end of this list, the country’s increasing population and lack of well-planned intervention strategies make it no lower diabetic risk than the US.
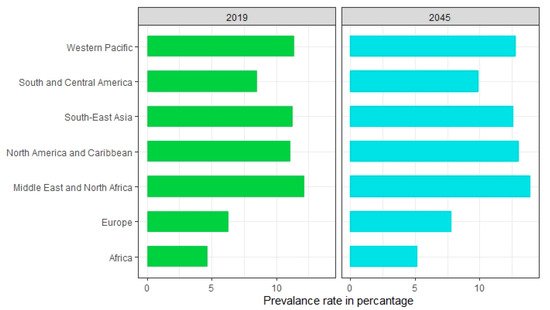
Figure 1. Prevalence of diabetes by world regions in 2019 and 2045 (estimated).
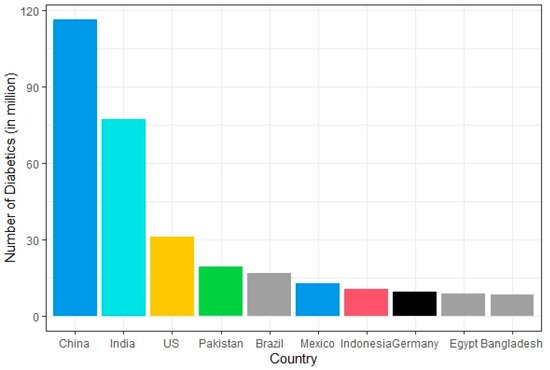
Figure 2. Countries with the highest number of diabetic patients worldwide in 2019.
3. Risk Factors for Diabetes
Genetics, atmosphere, loss of very first phase associated with insulin launch, sedentary way of life, lack of physical exercise, smoking, alcoholic beverages, dyslipidemia, reduced β-cell sensitivity, hyperinsulinemia, improved glucagon activity are the primary risk elements for prediabetes and DM [39,40,41,42,43,44,45,46]. These factors appear to play a significant role in insulin resistance or insulin nonfunctionality resulting in disease advancement. Most T2DM diabetic patients have often excess body weight. Obstructive sleep apnea and sleep disorder that are seen among overweight adult individuals are a common risk factor for insulin resistance and glucose sensitivity which collectively progresses to prediabetes and then T2DM. The diet containing low fiber but a high glycemic index (GI) is thought to be positively related to the onset of diabetes [47,48]. There is evidence that free fatty acids are one important link between insulin resistance and T2DM. Soft drinks of which the most deleterious feature is the high fructose load and consequent metabolic effects on the liver can cause obesity and increase body mass index (BMI) that may lead to T2DM. Some classes of drugs (i.e., antipsychotics, diuretics, immune suppressants, beta-blockers) also can induce diabetes [60,61]. Several infectious diseases including coxsackievirus, nonenveloped linear single-stranded RNA virus, rotavirus, and cytomegalovirus also trigger autoimmunity to T1DM. A couple of pancreatic diseases occur due to MMR (measles, mumps, and rubella) which can contribute to the development of T1DM producing autoantibodies [65,66]. Thus, diabetes can occur due to a complex interaction among many different risk factors.
4. Diagnosis of Diabetes
As untreated DM can lead to serious complications, early diagnosis of diabetes may prevent serious consequences due to the illness. Glycosylated hemoglobin (HbA1c) and oral glucose tolerance tests (OGTT) are commonly demonstrated for screening diabetes. Fasting plasma glucose test is another reliable routine method for the diagnosis of diabetes.
5. Complications of Diabetes
Long-term exposure to DM shows many other complications of diabetes. High blood glucose levels due to DM results in diabetic nephropathy, retinopathy, neuropathy, and diabetic cataracts. Ketoacidosis is common in diabetic patients due to the continuous production of ketone bodies [73]. T1DM patients may develop obstructive pancreatitis because of inflammation in the pancreas, hyperplasia of the pancreatic duct gland resulting in obstructive pancreatitis [75]. Diabetic patients are also at risk of free radical associated damage which is higher in diabetic patients than that of normal leading to atherosclerosis, cardiovascular disease, and hypertension. Prevalence of coronary artery disease (CAD), heart disease, and sudden cardiac death are elevated in diabetic patients. The high blood glucose level in diabetic patients stimulates superoxide production. Several studies indicated that cognitive dysfunction occurs in T2DM affecting intelligence, attention, memory, learning, and perception. Diabetes is also diagnosed as a potent risk factor for cancer because both share some common risk factors including age, sex, obesity, diet, smoking, and alcohol [14,76,77,78,79,80,81]. Other studies also indicated that different forms of cancer including liver cancer, pancreatic cancer, and non-Hodgkin’s lymphoma are predominant in diabetic patients.
6. Management of Diabetes
It was thought that once a patient is diabetic, he/she is diabetic for a lifetime; however, DM can go into remission [87]. Diabetes can be controlled by changing diet, doing physical exercise, maintaining reasonable body weight, monitoring lipid profile, and having appropriate medication when necessary. Moderate exercise which decreases obesity helps lowering blood glucose levels through insulin-independent glucose transport into the muscle. There are some drugs that are often prescribed to treat T2DM (Table 2). The most commonly used drugs are a class of biguanides, thiazolidinediones, α-glucosidase inhibitors, and glucagon-like peptide-1 agonist.
| Drug Class | Example | Mode of Action | Common Side Effects | References |
|---|---|---|---|---|
| Insulin secretagogues | Sulfonylureas | Inhibit β-cell K+ ATP channel and facilitate insulin secretion | Hypoglycemia | [111] |
| Meglitinides | Weight gain | |||
| GLP-1 agonists | Liraglutide | Increase glucose-dependent insulin secretion, reduces glucagon secretion, and delays gastric emptying | [112,113] | |
| Exenatide | Pancreatitis | |||
| Lixisenatide | ||||
| α–glucosidase inhibitors | Acarbose | Reduces the rate of digestion of carbohydrate in the intestine hence less glucose absorption | Abdominal pain | [114] |
| Miglitol | Diarrhea | |||
| Voglibose | Flatulence | |||
| Canagliflozin | Increases glucose excretion in urine | Hypotension | [115] | |
| SGLT-2 inhibitors | Dapagliflozin | Urinary tract infection | ||
| Empagliflozin | ||||
| Vildagliptin | Increases endogenous GLP-1 and GIP levels | Respiratory tract infection | [116,117] | |
| DPP4 inhibitors | Linagliptin | Nasopharyngitis | ||
| Saxagliptin | Headache | |||
| Sitagliptin | ||||
| Activate AMPK, decreasing glucose production and insulin resistance | Lactic acidosis | [118] | ||
| Biguanides | Metformin | Gastrointestinal irritation | ||
| Pioglitazone | Activate PPAR-γ, decreasing insulin resistance | Fluid retention | [119] | |
| Thiazolidinediones | Rosiglitazone | Weight gain |
7. Recent Technologies for Combating Diabetes
Advancement of stem cell technology has been a boon for researchers in developing a subsequent cure for type 1 diabetes [121]. Mesenchymal stem cell (MSC) therapy has shown considerable improvement in β-cell function in newly diagnosed type 1 diabetic patient while also showing considerable immunomodulatory effect [122,123,124,125,126,127]. Additionally, the pluripotent nature of embryonic stem cells (ESC) makes therapy using ESCs an appealing approach for treating type 1 diabetes [128,129]. Other promising diabetic treatment methods include VEGF inhibitors [131], SGLT2 inhibitors [132], SiRNA therapy [133], miRNA therapy [134], phytochemicals [135], and somatic gene therapy. While VEGF inhibition is not an absolute cure for diabetes, it has shown considerable effect against Diabetic Macular Oedema (DMO) [131,136], on the other hand, SGLT2 is a major renal protein responsible for glucose reabsorption, so SGLT2 inhibitors reduce the symptoms and risk factors of diabetes by lowering blood glucose level via inhibiting the glucose reabsorption pathway in kidneys [132]. Several studies have suggested several plants to carry antidiabetic properties too (e.g., Murraya koenigii, Allium sativum, Withania somnifera, Gymnema sylvestre, Allium cepa, Ferula foetida, etc.) [139,140,141,142]. In the case of somatic gene therapy, studies suggest this technique to have a considerable affinity for treating both type 1 and type 2 diabetes. While ex-vivo somatic gene therapy generates efficient β-cells, thus treating insulin deficiency [129,144], in-vivo somatic gene therapy prioritizes lowering blood glucose via generating both insulin [145,146] and noninsulin genes [147,148,149] (Gck, PTG etc.).
8. Future Perspectives
The finding of the relationship between diabetes and genetics, environmental factors, ethnicity, and lifestyle could be very helpful for the future development of the management of the disease. Changing lifestyle, doing sufficient physical exercise, maintaining body weight, controlling lipid profile, and minimizing glucose level are very effective. As uncontrolled diabetes could lead to serious complications, research focusing on the early detection of diabetes, developing various tools for understanding pancreatic β-cell destruction, developing targeted drugs are the appropriate steps to control the disease.
This entry is adapted from the peer-reviewed paper 10.3390/diabetology2020004
