Cryogels are interconnected macroporous materials that are synthesized from a monomer solution at sub-zero temperatures.
- injectable cryogel
- supermacroporous
- polymer
1. Introduction
Nowadays, the need for injectable biomaterials that have hemostatic effects in the field of tissue engineering and that are used in tissue regeneration is increasing. Furthermore, these biomaterials are preferred in order to minimize the risks and complications that may occur with surgical implantation. In addition to being biocompatible, three-dimensional (3D) scaffold biomaterials with physicochemical properties such as hardness, elasticity, and biological degradation provide a structural support and physical environment for cell attachment and subsequent tissue development. Cryogels, one of the biomaterials with injectable 3D scaffold structures, can be loaded with therapeutic agents or cells in line with the needs developing in clinical and tissue engineering studies. Thanks to their highly macroporous and interconnected structures, cryogels provide a suitable microenvironment for cell transmission, cellular infiltration, and neovascularization [1]. In recent years, the most widely used biocompatible polymeric biomaterials in biomedicine applications are cryogels.
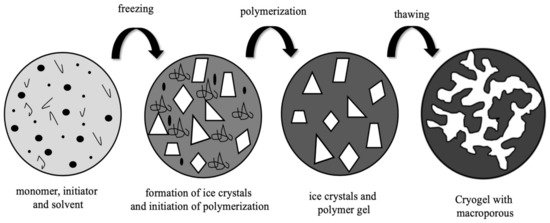
Cryotropic gelation is a gel formation process that is used to produce supermacroporous hydrophilic gels called cryogels. Cryogels are interconnected supermacroporous materials that can be synthesized at sub-zero temperatures using an existing activator/initiator pair [2,3]. Polymerization occurs in a frozen monomer solution in the interstitial spaces between ice crystals. Ice crystals act as a porogen throughout the scaffold during polymerization, and a supermacroporous polymer is formed after thawing [4,5]. Cryogels have important properties such as flexibility, large pores (10–200 μm), high mechanical strength, short diffusion paths, and good biocompatibility [6,7]. Cryogels can be synthesized in different forms such as powder, column, bead, sphere, membrane, monolithic, and injectable. Injectable cryogels with 3D porous structures are used for minimally invasive implantation in biomedicine applications, thanks to the advantages provided by their elastic structures, as well as their adjustable micron-scale and desired geometric structures. Injectable cryogels can be used in a variety of biomedicine applications, including diagnosis, therapy, drug delivery, and tissue engineering [8,9,10].
2. Cryogels
3. Injectable Cryogels
In recent years, injectable cryogels have been investigated for the minimally invasive implantation of 3D skeletons. The injectability of cryogels is a result of their elastic structure. These properties are very important for applications in soft tissue reconstruction when cryogels are injected subcutaneously. Cryogels have unique structural properties such as shape memory properties, degrees and mechanisms of crosslinking, interconnected macropores, and dense polymer walls [43].
Both micro- and macroscale injectable cryogels are used to provide a 3D porous structure, help protect encapsulated biological agents against degradation, and control the delivery of mammalian cells and biomolecules to host tissues. In addition, particle-based biomaterials, with their small size, can show insufficient retention at the injection site. This limitation increases the need for repeated injections, causing both serious side effects and increased costs. To reduce these limitations, macroscale cryogels are being investigated, as they can form a localized structure at the injection site [44,45].
With their shape memory properties and sponge-like macroporous structural features, injectable cryogels, which have similar properties to cryogels, can be easily recognized and accepted by the body with cell and purposeful ligand immobilization. For this reason, they have the potential to be the preferred biomaterial for many biomedical applications. These biomaterials have been used in many biomedicine applications such as immunotherapy, drug delivery, and tissue engineering. [27,46,47,48,49,50].
Biodegradable cryogels that provide temperature sensitive sol–gel transitions between body and room temperature are used in biomedicine applications. Biodegradation, which is an important element when preparing scaffolds for tissue engineering, is important for facilitating new tissue formation and integration upon implantation [51,52,53,54,55].
Macro-scale protein drug delivery systems are used to control the delivery of drugs to specific tissues. Careful material selection is often required for establishing an effective polymer drug delivery system for a particular protein, achieving the desired protein release profile, and maintaining bioactivity [56]. In drug-loaded cryogels, because the porous structure triggers the burst release of the drug, the use of a nanocarrier to overcome this situation could be a potential candidate for both controlling the drug release rate and enhancing the cryogels [57].
For many biomedicine applications, there is an increasing need for the tissue engineering of advanced 3D scaffolds to provide mechanical and structural support to tissue and cell regeneration. For this reason, 3D scaffolds should be made of resorbable and biocompatible polymers and have large macropores linked to each other [58].
Bone defects often require invasive surgery and are difficult to treat. Injectable cryogels are often used for bone tissue regeneration in the treatment of pathological fractures. Hixon et al. used injectable alginate-based cryogels with platelet-rich plasma (PRP). Although the use of PRP adversely affected the physical properties of cryogels, the freeze–thaw cycle improved their porosity and compressibility. These PRP-loaded, injectable cryogels have been shown to increase the proliferation and mineralization of human bone osteosarcoma cells. Injectable cryogel studies in biomedicine will be discussed in the following sections [59].
Improving the properties of injectable cryogels has provided promising results for biomedicine applications, and many methods are currently being studied in this regard. One of the major problems is insufficient control over the release of biomolecules, particularly low molecular weight components. Due to the high porosity in their structure, drug-loaded cryogels result in a burst release that limits their potential as a drug delivery carrier. Koshy et al. used injectable cryogels to improve drug release kinetics. Injectable cryogels combined with laponite nanoparticles (NPs) preloaded with immunomodulatory factors. Unlike freely injectable laponite cryogels, laponite NPs immobilized in injectable cryogels have been observed to inhibit burst release. In addition, the varying laponite content better adjusts the release kinetics from the cryogels while maintaining the injectability of the syringe [60].
Cryogels are reversible, collapsible, and elastic materials, and these properties make them suitable for injection. Generally, 16-gauge (16 G) is the preferred needle size for injecting 8 × 8 × 1 mm³. 16 G needles reduce penetration compared to surgical implantations, so the use of smaller needles will also reduce tissue damage and eliminate this problem. Therefore, optimization studies are required to improve the injectability of cryogels. While this size of injectable cryogel is used in biomedicine applications where large scaffolds are required, some optimization studies are carried out to avoid problems. In addition to the geometry and crosslinking mechanisms of injectable cryogels, it is necessary to optimize macrostructural properties such as pore size, pore connectivity, and flexibility. It is important to design injectable cryogels to prevent postoperative complications [10,61].
This entry is adapted from the peer-reviewed paper 10.3390/gels7020038
