1. Background
There are a lot of factors that limit the stability and performance of perovskites, like moisture, oxygen, light irradiation, which have been found and investigated by the research community. Niu et al. found that CH
3NH
3PbI
3 (MAPbI
3) could degrade in the presence of moisture and Al
2O
3 could successfully protect the perovskite layer [
10]. Aristidou et al. reported that MAPbI
3 photoactive layers rapidly decompose into CH
3NH
2, PbI
2, and I
2 after oxygen and light exposure, which is triggered by the reaction of superoxide (O
2−) with the CH
3NH
3+ cation from the perovskite absorber [
11]. Philippe et al. studied performance of both MAPbI
3-xCl
x and MAPbI
3 under higher temperatures. The perovskite films were heated in an ultra-high vacuum (UHV) chamber without the presence of air and moisture and degraded when the temperature approached 100 °C [
12]. Lee et al. observed UV degradation on PSCs after a 1000-h exposure of 365 nm UV light under inert gas at <0.5 ppm humidity without encapsulation [
13]. Nie et al. showed their PSCs formed light-activated meta-stable trap states under constant light illumination, which is caused by photocurrent degradation [
14]. In addition to the MA-based perovskite, Formamidinium (FA) based perovskites have been reported more stable than pure MA-based perovskites under high temperature, as the molar mass of FA is larger than MA [
15,
16]
There are also investigations on other factors, such as Iodine vapor, voltage, device structure and mechanical stress [
17,
18,
19,
20,
21]. On device level, Eperon et al. found that FAPbI
3 substrate showed higher stability as compared to MAPbI
3 when both were heated at 150 °C [
15]. Lee. et al. showed that oxygen does not harm the stability of the PSCs as they were stored in the dark [
22]. The most commonly used hole transporting material, spiroOMeTAD is unstable in the presence of water which could cause the decomposition of the PSCs [
23]. Leijtens et al. observed that PSCs tend to degrade under light illumination which could decrease short-circuit current density (J
sc) [
21]
To improve the stability and the performance of the PSCs, it is essential to have a better cognition not only on the device level but also on the material level. However, most previous stability works were based on the observation of device performance, which is usually focused on the parameters including open-circuit voltage (V
oc), J
sc, fill factor (FF), and PCE [
24,
25,
26,
27,
28]. These parameters are often monitored in a function of time to measure the stability of the device. The reduction of these parameters usually results from the degradation of the perovskites, but the degradation mechanism did not receive attention as much as the device performance. Therefore, the study of the surface level of the electronic and chemical properties of the perovskites is necessary to help develop possible solutions for the stability issues.
There is a number of choices of method to grow perovskites: spin coating, spray coating, chemical vapor deposition (CVD), physical vapor deposition (PVD), thermal evaporation, blade coating, and screen printing [
29,
30,
31,
32,
33,
34,
35]. Spin coating is a simple method to fabricate thin films and is widely used to quickly and easily synthesize perovskites in a lab environment [
27,
36,
37,
38,
39,
40]. The PSC with high efficiency of 24.8% was achieved with spin coating method [
41]. Besides, it has been applied to fabricate large-scale perovskite photovoltaic devices [
42]. However, a more suitable way for environmental tests is thermal evaporation. Studies showed that evaporated perovskite films have a more uniform surface, fewer defects, and more ideal chemical stoichiometry [
43,
44,
45,
46,
47,
48]. Besides, the thin films are prepared in the vacuum system which can be processed for the measurements right after the film growth without being exposed to the ambient environment. Similar to evaporated thin films, the in situ cleaved perovskite single crystals (SCs) are also suitable for the degradation measurements [
49]. Crystallinity plays an important role in the degradation process. Since the disorder of material was increased by the defects on surfaces, voids, and grain boundaries thus weaken the bonding, degradation would be more severe where the defects exist. In this case, studies show that moisture and thermal stability of the perovskite material can be increased by the surface and grain boundary passivation [
50,
51]. Therefore, better crystallinity is preferred for the studies on degradation.
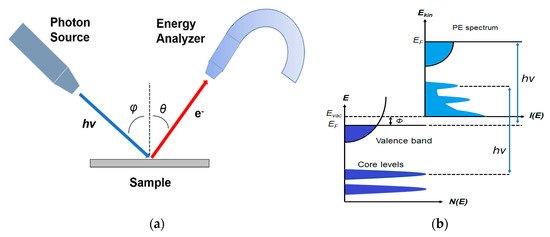
Photoemission spectroscopy (PES) is one of the most suitable and widely used techniques to probe the chemical and electronic properties of a material [
52]. As explained by Einstein in 1905, photoelectrons are generated by the interaction between the irradiating photons and the sample [
53]. The schematic of PES is shown in a. The incident photons excite electrons which can be detected by an electron energy analyzer, if the excited electrons have enough energy to leave the sample and escape to the vacuum. The kinetic energy of the excited electrons is
EK, and it is expressed by the following equation [
54]:
where
hv is the photon energy,
EB is the binding energy (BE) of the electron with respect to the Fermi level, and the work function is represented by
Φ which is the minimum energy needed to move an electron from solid to the vacuum.
Figure 1. (
a) Schematic of a photoemission experiment. (
b) Schematic representation of photoemission spectroscopy (PES). From Ref. [
47] with permission.
The schematic of PES is shown in b, which demonstrates that PES detects photoelectrons from occupied states, including the valence bands (VB) and the core-level states, depends on the photon energy it uses. Also, the vacuum level cutoff is defined by the secondary electrons excited by the photoelectrons [
55].
PES is highly surface sensitive as a surface analytic tool, which uses photoexcited electrons as the probing particles. These electrons have a strong interaction with the solid, resulting in a shorter escape distance, known as the mean free path (MFP). The MFP is the average distance that the particles can travel before colliding with another particle, which is highly determined by the kinetic energy of the photoelectron and has less material dependency [
56]. The thickness of a monolayer is typically ranging from 0.2–0.4 nm in different materials.
X-ray photoemission spectroscopy (XPS) is a most commonly used PES technique to investigate the performance of semiconductor materials [
57]. MFP of XPS is less than 10.0 nm and as an in-situ technique with high surface sensitivity, it has been widely used to monitor compositional change on the surface which is caused by degradation [
58,
59,
60]. Detailed control of the exposure measurements is able to be performed within the UHV system.
3. Environmental Stability of Perovskite Single Crystals
3.1. X-ray Exposure on MAPbBr3 Single Crystal
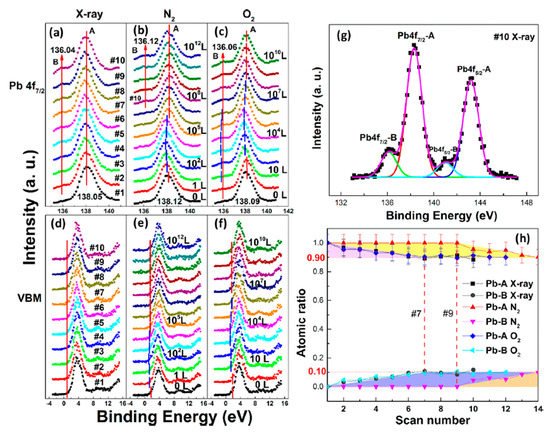
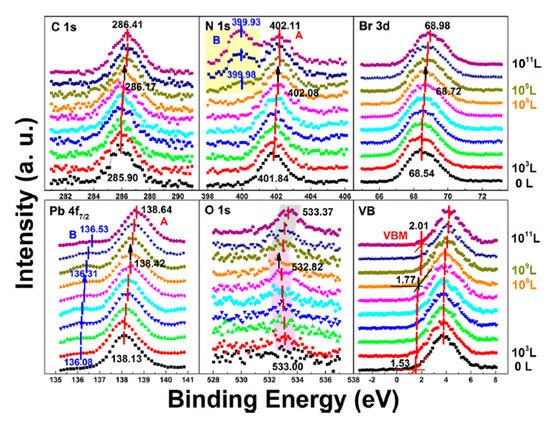
In a, a second Pb component (Pb-B) started to show up at ∼136.04 eV from the second scan as marked by the red arrow, which is identified as the metallic Pb, while the perovskite Pb-A peak is at ∼138.05 eV. As shown in , the core level of all elements has no significant change, except for Pb.
Figure 6. (
a–
f) XPS spectral evolutions of Pb 4f7/2 and VBM of cleaved MAPbBr3 single crystal under X-ray, N2, and O2 exposures, respectively. (
g) The detailed fitting curves for Pb under the 10th X-ray scan. (
h) Elemental ratio comparisons of Pb peaks under the three conditions with error bars. From Ref. [
38] with permission.
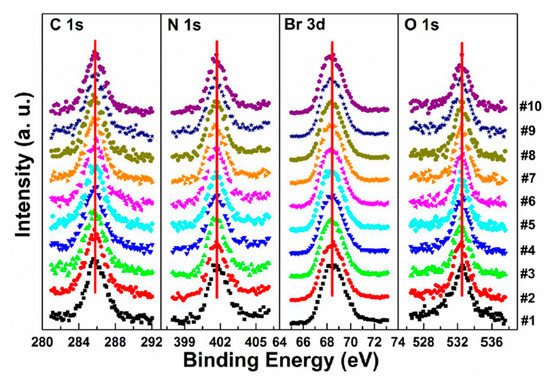
Figure 7. Evolutions of C 1s, N 1s, Br 3d, and O 1s XPS spectra of cleaved MAPbBr
3 single crystal under X-ray exposure. From Ref. [
38] with permission.
A possible reaction is proposed to explain the conversion of metallic Pb under X-ray exposure [
49].
By comparing the elemental ratios of the whole process, 10% Pb-A converted to Pb-B, which suggests that 10% of the perovskite decomposed after 10-h X-ray exposure. C 1s peak position is ∼285.81 eV and no amorphous carbon showed up at ∼284.6 eV at either the test spot () or the control spot (
Figure S1 (see supplementary materials)). It shows that the increased carbon was not from the contamination in the UHV chamber, which may suggest that it could be attributed to the surface diffusion of MABr.
3.2. Nitrogen Exposure on MAPbBr3 Single Crystal
Although N
2 is normally used to protect the sample as an inert gas during the perovskite environmental studies [
66,
88,
89,
90]. the role of N
2 in the degradation process remains to be clarified.
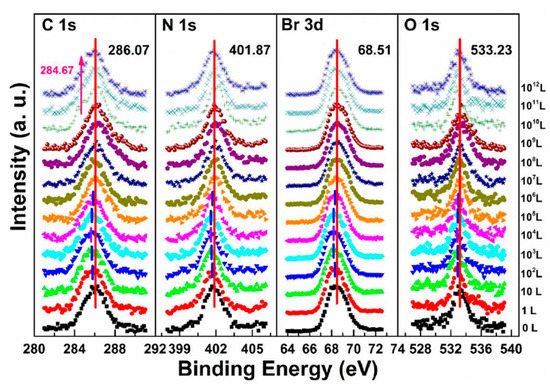
b,e and are associated with N2 exposure. Before 107 L, it can be observed that all peaks and VBM started to move toward the low BE direction and reach the maximum at 102 L by about 0.31 eV, then shifted back to the original position at 105 L. This represents that the perovskite crystal was slightly p-doped by nitrogen via physical absorption and N2 slowly left the surface afterward. There is no metallic Pb nor other elemental ratio change at 107 L with 9-h X-ray exposure, suggesting that the sample surface can be protected from X-ray degradation with N2 for up to 9 h and stop MABr diffusion at the same time. However, metallic Pb peak started to show up at 108 L and increased to 10% of the total Pb at 1012 L (14-h XPS scan). The degradation process is the same as discussed in Equation (5). It can be concluded that N2 could protect perovskite crystal from XPS X-ray degradation for 9 h as it p-doped perovskite by ∼0.31 eV first then slowly left the surface.
Figure 8. Evolutions of C 1s, N 1s, Br 3d, and O 1s XPS spectra of cleaved MAPbBr
3 single crystal under N
2 exposures. From Ref. [
38] with permission.
3.3. Oxygen Exposure on MAPbBr3 Single Crystal
In the previous discussion of perovskite thin films, O2 does not react with the sample and only acts as a p-dopant during the exposure. It’s also important to know if it acts the same with perovskite SCs.
The metallic Pb peak began to develop from the second scan as under the X-ray exposure, which suggests that O
2 cannot shield the sample as N
2 did under the same condition, as shown in c,f. The peak position and the ratio changes indicate that the exposure process consists two steps. In step one, all peaks started to move to the lower BE direction from 1 L and reached the maximum at 10
4 L, except O 1s. The VBM also had the same pattern with a maximum shift of ∼0.18 eV, which is the same as the oxygen exposure on perovskite thin films [
47]. Meanwhile, O 1s started to move to the higher BE region from the beginning and achieved the maximum of ∼534.09 eV at 10
4 L. These firmly suggested that the detected O 1s mostly came from the crystal surface by O
2 p-doping.
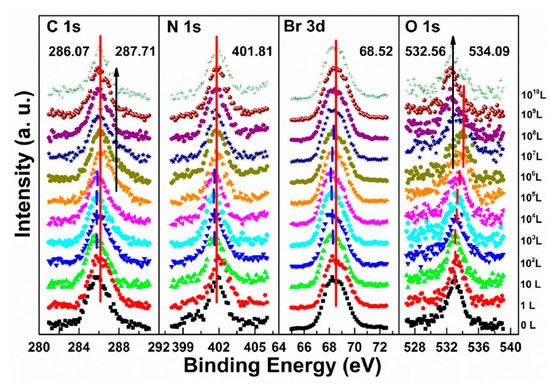
shows that a second carbon peak at ∼287.71 eV (C 1s-B) and a second oxygen peak at ∼532.56 eV (O 1s-B) appeared after 104 L in step two, which indicates the doped oxygen started to bond with carbon and formed C−O bond, at 1010 L. Other peaks began to move back to their original positions as the doped O2 decreased. It is noted that carbon which bonded with oxygen came from MABr due to X-ray degradation and also from MA+ diffusion. Similar to perovskite thin film, perovskite SC also does not react with O2.
Figure 9. Evolutions of C 1s, N 1s, Br 3d, and O 1s XPS spectra of cleaved MAPbBr
3 single crystal under O
2 exposures. From Ref. [
38] with permission.
By comparing the metallic Pb ratios from the three atmospheres, it can be found that no matter when the degradations started and how long the exposures were, the ratio of the metallic Pb always saturated at 10%. This may be because that the perovskite surface was covered by the 10% metallic Pb layer which prevented it from further decomposition. It has been reported that MAPbBr
3 single crystal has better stability and can be stored in air for eight months [
91,
92,
93]. In addition, oxygen exposure could enhance photoluminescence (PL) performance of the MAPbBr
3 single crystal [
94].
3.4. Water Exposure on MAPbBr3 Single Crystal
Similar to the perovskite thin film, perovskite SC may also vulnerable to moisture. shows the evolution of five major elements and the VB region. The whole exposure process can also be divided into two stages.
Figure 10. Evolution of C 1s, N 1s, Br 3d, Pb 4f
7/2, O 1s, and the VB region of cleaved MAPbBr
3 single crystal under water exposure from 0 to 10
11 L. From Ref. [
38] with permission.
In stage one, there was a rigid shift of ∼0.24 eV to the higher BE direction for carbon, nitrogen, bromine, and lead peaks before 108 L. The rigid shift indicates that water worked as an n-dopant, causing the Fermi level of the crystal to move from 1.53 eV to 1.77 eV. The metallic Pb peak appeared at ∼136.08 eV from the second scan which was caused by X-ray degradation. There is no obvious signal for oxygen from the freshly cleaved sample and the oxygen peak started to show up at ∼533.00 eV from 103 L, which can be attributed to water. Then it moved toward the lower BE direction with a maximum of ∼0.18 eV at 108 L. This displacement in opposite direction indicates that there was no chemical reaction between water and the SC during this stage.
The ratio is C/N/Pb/Br/O = 1.53:1.10:1:2.92: for the freshly cleaved MAPbBr
3 SC. The same 10% ratio of Pb from perovskite converted into metallic Pb at the end of the first stage (10
8 L of H
2O), as the net change under 7 h exposure of X-ray. However, the ratios of C 1s, N 1s, and Br 3d
5/2 dropped by ∼30, 37, and 27%, respectively, and doped water caused a 14% increase in O 1s. In addition to Equation (5), the following mechanism is proposed for step one in water exposure [
49].
There is ~30% MABr from the diffusion and the X-ray degradation further decomposed into CH3NH2 and HBr gases under X-ray with 14% doped water and then escaped the sample surface.
In the second stage, MAPbBr3 SC started to react with water from 109 L as two noticeable changes are shown in . First, the O 1s quickly moved to the higher BE direction by ∼0.55 eV, which was the same for the other major peaks but opposite from that before 108 L. The oxygen peak at ∼533.37 eV (1011 L) may come from hydroxide. C 1s, Br 3d5/2, two Pb peaks, and VBM all shifted ∼0.24 eV to the higher BE during the period. Second, there was a second nitrogen peak (N 1s-B) showed up at ∼399.98 eV, which may be caused by NH4+ formed by the reaction of NH3 and H2O. It slightly shifted ∼0.05 eV to the lower BE direction, while perovskite nitrogen peak N 1s-A kept moving to the higher BE until achieving ∼402.11 eV at 1011 L. These results strongly demonstrate that water was a key factor causing decomposition of perovskite SC in the second step. After the exposure, Fermi level was moved to 0.29 eV, as the bandgap is 2.3 eV for the MAPbBr3 SC, which indicates a very n-type doping.
Metallic Pb increased 17% while the saturate ratio is only 10% under the same X-ray exposure condition. The extra metallic Pb may attribute to X-ray degradation of PbBr
2, which is from MAPbBr
3 and water reaction. After 10
10 L, carbon increased ∼40%, while nitrogen and bromine decreased ∼39 and 32%, respectively, exposure level. Therefore, the following reactions were proposed in stage two [
49].
This degradation mechanism is very similar to Equation (2). of the MAPbI
3 thin films. About 35% perovskite was decomposed into HBr and NH
3 gases by water in UHV. Partial ammonia was absorbed by water, while PbBr
2 was further decomposed into metallic Pb and Br
2. From 10
10 L, white precipitate Pb(OH)
2 was formed by the reaction of metallic Pb, water and the residual oxygen in the chamber. Bulk crystal could be intact with some water molecules and reversible photoelectrical properties [
94]. However, PL confirmed the doping of the H
2O molecules, thus leading to the lattice distortion-induced reconstruction of the SC [
95].
3.5. Light Exposure on MAPbBr3 Single Crystal
The stability of MAPbBr
3 SC under light illumination was investigated with a blue laser, which has a wavelength of 408 nm and intensity is ~7 times the AM 1.5 irradiation. The exposed spot received a total light exposure time of 44 h. Strong chemical decomposition was observed with XPS after the light exposure. It has been reported that structural changes in the perovskite lattice were observed under illumination, which is related to the light-induced ion migration and associated defect passivation [
96]. Anaya et al. reported that by combining in situ PL and XPS analysis, they found the formation of a negatively charged layer of adsorbed anionic oxygen species on the surface which could drive halide anions away from the illuminated areas toward the bulk of the material [
97].
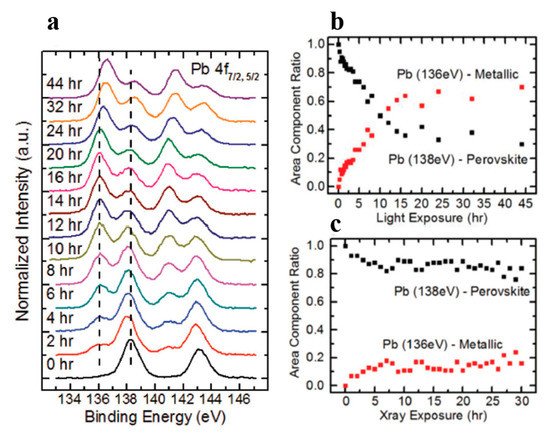
A new metallic Pb spectral component started to show up in the Pb core level spectra as shown in . The original perovskite Pb 4f7/2 and 4f5/2 peaks were located at 138.27 and 143.17 eV, respectively. A new metallic Pb feature started to appear for each Pb core level after light exposure. Both the metallic and the perovskite Pb peaks moved to a lower BE which occurred within the first hour of light exposure. C, N, and Br peaks also had a similar initial BE movement as shown in . This rigid shift is corresponding to the Fermi level movement within the bandgap, which suggests the perovskite was p doped.
Figure 11. (
a) A stack plot of the Pb 4f
7/2 and 4f
5/2 core levels of cleaved MAPbBr
3 single crystal with increasing light exposure. (
b,
c) The ratio of perovskite Pb to metallic Pb under light exposure and X-ray exposure, respectively. From Ref. [
62] with permission.
Figure 12. (
a) A stack plot of the C 1s, N 1s, Br 3d
5/2 core levels of cleaved MAPbBr
3 single crystal with increasing light exposure. (
b) Elemental ratio change for C, N, and Br under light exposure. From Ref. [
62] with permission.
After the initial BE shift, all peaks started to gradually shift back to higher BEs and the metallic Pb peaks stopped at ~136.6 and 141.4 eV for the 4f7/2 and 4f5/2 core levels, respectively. As the light exposure progressed, the metallic Pb component continued to gain in intensity and finally dominated the overall Pb core level after ~10 h of exposure. When the whole 44 h exposure was finished, ~70% of Pb signal came from the metallic Pb, while only ~30% signal was the perovskite Pb.
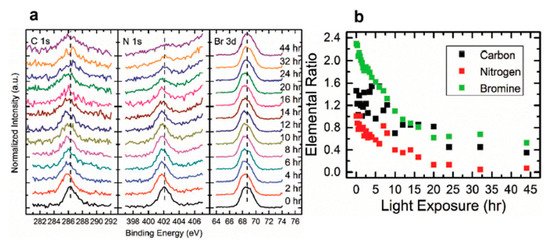
For all other elements, there were no new features observed during the exposure, which suggests that they did not change their chemical states. However, the surface saw noticeable concentration losses in C, N, and Br. In , at ~10 h of light exposure, C, N, and Br lost about half of their initial concentration, and ~50% of the perovskite Pb was degraded into metallic Pb. Based on these observations, the following mechanism was proposed to explain the degradation process [
98].
The perovskite SC first degraded into MABr and PbBr
2, then PbBr
2 further degrade into metallic Pb and Br
2 under light exposure. C, N, and Br were decomposed as volatile species, then escape the sample surface similar to simple outgassing. Interestingly, a similar degradation process was also observed under E-Beam Irradiation [
99].