Using adhesives for connection technology has many benefits. It is cost-efficient, fast, and allows homogeneous stress distribution between the bonded surfaces.
- adhesive
- surface pre-treatments
- sustainability
1. Introduction
1.1. Overview
Adhesively-bonded joints (ABJs) consist of generally two joined components and the layer of adhesive between them [1][2]. The most important part of the ABJ is the adhesive itself, that is, the component depending mainly of the formulation, followed by the preparation process that precedes the application of the adhesive. Adhesive bonding requires a special manufacturing process, which involves three main aspects: qualified methods/processes, trained operators, and dedicated tools.
Complex and advanced technologies, or series of technologies, have arisen to enable the application of adhesives in many fields. The diversity of substrates and the continuous development and introduction of new processes and materials has placed the adhesives technology as one of the most swiftly expanding manufacturing endeavors.
As in any emergent technology, strict environmental regulations, such as those emitted by the European Chemicals Agency (ECHA), including REACH (Registration, Evaluation and Authorization of Chemicals) compliance, play an important role, which dictates products certification and commercialization. These aspects are affecting the sector, driving a marked growth in consciousness to reduce fossil dependence and mitigate global pollution, which result in an increasing demand for products manufactured from renewable and sustainable sources.
As for the future of adhesives, since they are polymeric materials, the recommendations toward sustainability are: understanding their impact throughout product lifecycle, developing new sustainable polymeric materials, closing the loop of plastics recycling, and understanding and controlling plastic degradation [3]. Developments in chemistry will be critical to understanding and mitigating the impact of plastics in the environment.
This paper complements other reviews in the topic [4][5][6][7][8][9][10], by giving an overview of eco-friendly emergent adhesive technologies, surface treatments that precede the application of adhesives, new trends in adhesive waste management, including recycling and design for disassembly, and introduces new insights on the connection between Research & Development (R&D) efforts, industry standards and regulatory aspects, which unquestionably influence the roadmap of adhesives chemistry development.
1.2. Well-Established Industrial Adhesives
The adhesive crucial role is to transfer the load from one base material to the other. To date, a wide array of adhesives found practical application and were tested experimentally, in order to find the most suitable way of creating ABJs between a wide spectrum of different engineering structural materials (substrates), ranging from metals and alloys, composites, to natural materials as different types of wood.
Adhesives and sealants can be classified in several ways: by chemical composition—adhesive binder, natural vs. synthetic, organic vs. inorganic, structural vs. nonstructural, curing or setting mechanism, etc. [11]. However, the most common classification is based on the adhesive binder, as listed in Table 1.
|
Adhesive |
Properties |
Applications (Adherends) |
|
Epoxy |
High strength and temperature resistance, good durability and resistance to environmental extremes, relatively low cure temperatures (for two-component formulation (2K)), easy to use, low cost. |
Most materials, metals, ceramics, polymers |
|
Acrylics |
Versatile (design flexibility), high strength, fast curing, tolerates less prepared surfaces |
Cloth, plastics, metals |
|
Polyurethanes |
Good flexibility at low temperatures, resistant to fatigue, impact and durability, ideal for creating strong flexible bonds between dissimilar materials. |
Plastics, metals, rubber |
|
Cyanoacrylates (superglues) |
Fast bonding capability to plastic and rubber but poor to moisture and temperature |
Almost any adherends |
|
Anaerobics |
Fastening and sealing without light, heat or oxygen, suitable for cylindrical shapes |
Metals |
|
Silicones |
Excellent sealant for low stress applications, high flexibility, very high temperature resistance, long cure times (for one-component formulations (1K)), low strength |
Metals, glass, paper, plastics, rubber, fluorocarbons |
|
Phenolics |
Good strength retention for short periods of time, limited resistance to thermal shock, low cost |
Metals, wood |
|
Polyimides |
Thermal stability, dependent on a number of factors, difficult process ability, expensive |
Cloth, plastics |
|
Bismaleimides |
Very rigid, low peel properties |
Metals, glass, ceramics, plastics |
|
Amino resins (e.g., urea-formaldehyde) |
High strength, rigidity, cost effectiveness, and fast cure |
Wood |
Moreover, different adhesive technologies can be categorized by increasing order of load bearing capability, typically from ca. 0.01 to 40 MPa of overlap shear strength, as follows: pressure sensitive adhesives, reclosable fasteners, contact and spray adhesives, acrylic foam tapes, hot melt adhesives, adhesive sealants based on polyurethane and hybrids, polyurethane adhesives (PUR), epoxy, and acrylic and urethane structural adhesives. Structural adhesives provide several advantages such as strong bonds, design flexibility and process efficiency. Well-established industrial adhesives encompass different chemistries, curing methods, open times and final bond capabilities, including low-odor and non-flammable versions of certain chemistries to meet specific regulatory and safety requirements.
1.3. Opportunities and Challenges in the Adhesives Sector
ABJs are commonly used in a wide variety of applications. Some estimations claim that over 20% of ABJ are used in construction (windows, doors, pipes, flooring, insulation, glazing, tiles, among others). However, other applications have emerged, such as those in the fields of electronics, energy, marine, automotive, and aerospace industries [2][10][14].
1.3.1. Replacement of Mechanical Fasteners by Adhesives: A Reality?
Although adhesive bonding technology has shown potential for several decades in the aerospace industry, its application as a joining technology and assembly method frequently occurs in secondary parts of the aircraft structures. Typically, in primary structures, different parts of aluminum sheets, being the most common ones, AA2024-T3 and AA7075-T6 (bare and cladded materials), are assembled relying on fasteners, namely rivets. Indeed, recent developments in the automatization have made riveting economically attractive, however, this bonding technology exhibits some limitations.
Some of the advantages of ABJs vs. alternative joining techniques such as mechanical fastening methods as riveting, especially in aerospace applications are shown in Table 2.
Table 2. ABJs’ advantages and disadvantages [1][12][15].
|
Adhesively Bonded Joint Advantages |
Adhesively Bonded Joint Disadvantages |
|
Expressive weight savings, as there are no rivets (points of stress concentration) |
Requires surface preparation—thorough cleaning/degreasing |
|
More evenly distributed stresses |
Curing times can be significant |
|
Cost savings: hole fabrication is not needed |
For curing, heat and pressure may be needed |
|
Excellent fatigue resistance |
Strength consistency is highly dependent on rigid process control |
|
Increased vibration and shock resistance |
Conventional techniques of non-destructive inspection of ABJs is difficult |
|
Increased compliance to critical tolerances |
Adhesive shelf-life is limited; thus, special storage conditions are required |
|
Provides a way to seal the entire bonding area |
Lower humidity and temperature resistance |
|
Enables joining of dissimilar base materials |
|
|
Smooth contours and sections around joint areas |
As a consequence of the noted advantages of adhesives vs. other means of joining, the application of ABJs has grown significantly in recent decades, with a good prospect to become even more attractive in the future [12]. For instance, when joining composite components, mainly epoxy and polyurethane (PU) adhesives are used. They are characterized by high strength and stiffness values at high elongation levels. This results in very positive impact and fatigue properties of bonded joints.
Nowadays aircraft manufacturers are increasingly using carbon fiber-reinforced polymers (CFRP) as a lightweight and robust replacement of aluminum. Riveting such composite materials has a lot of hazards, such as fiber breaking, stress concentrations and black dust. Adhesive bonding is the most promising joining technology in terms of lightweight and performance for assembling composite parts. However, the status in the aircraft industry is that bonded joints without additional fasteners are only certified for assembling secondary structures of an aircraft, whose failure is not detrimental for aircraft safety. In comparison with metals, composites bring extra challenges, since the manufacturing processes use release agents, which can reduce adhesion strength, which cannot be predicted with the conventional non-destructive testing, such as ultrasound. New alternatives are urgently needed to use the full potential of bonded systems in composite aircraft structures. Efforts are being done in this sense, such as European collaborations, namely the COST project CERTBOND—“Reliable roadmap for certification of bonded primary aircraft structures” (certbond.eu), which addresses this need, by tackling the scientific challenges in the different stages of the life-cycle of a bonded structure.
Interfacial failure is a critical aspect that must be avoided during the in-service life of bonded structures since it is unpredictable. It is proved to be connected to the lack of chemical bonding between the bonded surfaces. It is hence believed that the next breakthrough in the adhesive bonding technology requires the fully integrated research and development, from the basic chemistry up to the final design and manufacturing. Only such a holistic development and suitable product design can provide the required reliability to be demonstrated for aerospace certifications.
1.3.2. REACH Evolution and Its Impact on Adhesives Industry
Replacing hazardous chemicals and manufacturing processes by safer chemicals and greener technologies has been a key investment of adhesive companies, to avoid regrettable roadblocks in product commercialization in a near future. In addition to the benefits to companies, the environment, and the health of workers and consumers, this can also have a significant positive impact on the implementation of a circular economy. The continuous evolution of REACH regulation brings a critical challenge in what concerns requalification of new adhesive versions and upgrade of safety equipment and procedures. In some cases, it leads to product obsolescence and the stop of production lines if adequate measures are not duly implemented.
An example of REACH compliance is herein given for the case of bicomponent polyurethane formulations. Component B consists of 4,4′-methylenediphenyl diisocyanate (MDI), which, despite being considered less hazardous than other isocyanates (e.g., toluene diisocyanate—TDI) it is classified as Harmful (Xn) by ECHA, namely irritant for skin, eyes and respiratory organs. It is also suspected to cause cancer and severe damage to organs through prolonged exposure. According to Annex XVII of REACH (Council Directive 76/769/EEC of the European Union), the restrictions for MDI involve the use of this compound only by qualified personnel with no asthma, eczema, or allergic reactions to isocyanates, in well-ventilated areas and with appropriate protection equipment. In addition to selecting less hazardous isocyanates, companies, such as Henkel, have released improved formulations, such as TEROSON PU 6700 ME (MicroEmission technology) which is a solvent-free polyurethane bicomponent product, with very low volatile organic compounds (VOCs), with no R40 (limited evidence of a carcinogenic effect) risk phrase due to micro-emission property.
Another example of REACH impact on adhesives industry is for epoxy adhesive formulations, as some of the main current epoxy-based precursors are classified as H341, i.e., suspected of causing genetic defects, namely triglycidyl-p-aminophenol (TGPAP) and N,N′-tetraglycidyl diaminodiphenylmethane (TGDDM). On the other hand, amine-based precursors, such as 4,4′-diaminodiphenylsulfone (44DDS) are in the scrutiny for being possible endocrine disruptors (included in the Community rolling action plan, CoRAP) and trimellitic anhydride is classified as a substance of very high concern (SVHC). Moreover, widely employed organic solvents in these adhesives, such as n-methyl-2-pyrrolidone (NMP), are classified as H360, i.e., may damage fertility or the unborn child, are classified as SVHC and included in the Candidate list and restricted under Annex XVII of REACH. NMP’s production, use and sale are forbidden, as substance or as component of mixtures in concentration equal or greater than 0.3%, since May 2020 (Reg. 2018/588).
Another substance also quite common in adhesives formulations, mainly used for wood-based panels, is formaldehyde, which is carcinogenic, suspected to be mutagenic and skin sensitizer. It is restricted under Annex XVII of REACH (Reg. 2018/675) since 2018.
Inclusion in the Candidate List can trigger certain legal obligations for the importers, producers and suppliers of an article that contains such a substance. Thus, there is plenty of room for the identification and development of greener, non-hazardous chemicals that comply with safety and environment regulations and enable the required performance of adhesives.
1.3.3. Adhesives from Renewable Resources
The production of the above-mentioned resins has relied on non-renewable petroleum resources. Due to the growing demand of environmentally friendly, green and sustainable materials, as well as economic and availability issues of petroleum resources, there has been a growing interest in the development of environmentally friendly adhesives from renewable resources. For the sake of clarity, a biopolymer is a naturally occurring polymer, such as cellulose or starch, while a bio-based or bio-derived plastic is a polymer that is produced from biological resources, including chemicals derived from plants and algae. e.g., polylactide is produced from sugar, which is harvested from plants like sugar cane [3].
Indeed, the first uses of bio-adhesives widely preceded the use of synthetic adhesives, since they date back from the middle and upper Paleolithic period [16]. Although the effectiveness of natural adhesives (biopolymer) was satisfactory for those times, they are all but efficient and well suited to modern production demands [17]. However, due to advances in polymer synthesis, several man-made, mass produced adhesives suitable for joining of different base materials have emerged and found practical application in ABJs. Currently, there is a wide variety of eco-friendly adhesives and sealants, based on cellulose, starch, lignin, vegetable oil, and protein-based silanes, which are yet mainly employed for non-structural applications. Investment and advances on biopolymer production and processing can lead to a decrease of manufacturing costs, while creating new “green” businesses opportunities [4][10].
2. Eco-Friendly Emergent Structural Adhesives
The desire for cleaner and healthier environments, and the evolving regulatory aspects in this direction, are promoting the replacement of petroleum-based raw materials with natural components, and, at the same time, limiting the use of non-renewable resources for adhesive formulations. The concept of the circular economy was created in order to optimize a more efficient consumption of products while making people more aware of the anthropogenic activities that are related to high levels of pollution and waste products [18]. From another perspective, bio-based materials have gained significant attention in almost all fields due to the well-known issues that regard the use of petroleum-based materials [4][19][20][21][22]. These trends are also noticed in the field of adhesives, where the production of monomers from bio-renewable resources and waste products have been comprehensively investigated. This section emphasizes the importance of bio-based raw materials, polymer recycling, and the possibility to design “greener” formulations for structural adhesives which contribute to efficient waste management, economic benefits, and environment/health protection.
2.1. Renewable/Bio-Derived Adhesives
Efforts have been made in adhesive technology toward the use of renewable materials to produce commercial counterparts with equal or even better performance. Using renewable resources in adhesive formulations is not, per se, necessary and sufficient reason for commercialization. Market penetration might be particularly difficult if associated additional costs are envisaged and if a straightforward drop-in substitution for the present technology is not provided. Moreover, it will depend if existing and new biopolymers companies are able to increasingly adopt highly efficient, continuous production technologies and if an effective channel of increased knowledge creation and transfer to the industry is created. Commercialization of bio-based adhesives has another benefit in terms of circular economy, regarding their straightforward reutilization since they are more prone to biodegradation. The presence of ester linkages, for instance, present in many biopolymers is known to promote biodegradability [23][24][25]. This chapter will show the advantages of adhesives derived from varied bio-sources, designed for wide spectrum of applications and opportunities.
2.2. New Trends in Polymer Waste Management for Adhesive Formulations
Currently, most of the structural adhesives are synthetic polymers obtained from non-renewable fossil fuel resources. Moreover, the exploitation of fossil fuel is associated with pollution and the continuous increase and release of “greenhouse gases” into the environment. Compared to classic fasteners, structural adhesives are preferred by the automotive and aerospace industry considering the advantages that they bring in terms of mechanical and technological requirements [26], as well as lighter weight. Unfortunately, difficulties in the recycling methodology can emerge, due to the small portion of adhesive in the final components, and the fact that using polymers for adhesive bonds produces complex material combinations that are difficult to separate and, hence, recycle. ABJs are designed for high adhesion performance and their destruction and removal from the substrates are nearly impossible [27][28], which poses some difficulties in maintenance or recycling of bonded materials. It should also be stressed that there is a trend in what regards the use of thermoplastic-based adhesive formulations, since they can be recycled or reused by re-melting, contrary to thermosets, which are typically used for structural parts and are currently not being recycled. In this sense, providing structural properties to thermoplastic-based adhesive formulations becomes the challenge.
In 2018, global plastics production almost reached 360 million tonnes (metric tons), while in Europe it almost reached 62 million tonnes [29]. The resulted plastic waste is treated differently using various strategies such as incineration, landfill disposal, and recycling (Figure 1) [30]. Landfill disposal has been banned in several countries. In the European Union, the incineration of plastic waste for energy recovery represents almost 40% of all strategies, which produces, annually, millions of tonnes of CO2 emissions [31]. Considering these aspects, some new trends in adhesive formulations are currently related to plastic and polymers waste recycling policies in order to ensure the circular economy “closing-loop” principle [28].
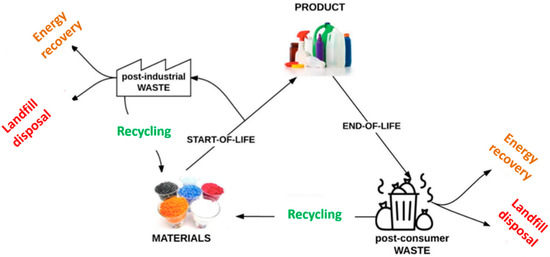
Figure 1. Lifecycle and routes for energy recovery, landfill disposal and recycling of plastic materials (Adapted from [31]. Reprinted from Waste Management, Vol 69, Ragaert et al., Mechanical and chemical recycling of solid plastic waste, 24–58. Copyright (2017), with permission from Elsevier).
According to the document A European Strategy for Plastics in a Circular Economy released by the European Commission in 2018, a recycling rate increase to 50% of the total plastic waste must be reached by 2030 [32]. Based on these recycling policies, the tendency in structural adhesives is to apply chemical or mechanical recycling processes to plastic/polymer waste to valorize them to raw materials for new formulations.
Poly(ethylene terephthalate) (PET) is one of the most abundant polymers used for packaging or beverage industry. Until now, the continuous manufacturing of PET products led to the accumulations of large amounts of plastic wastes (51 wt% from the total amount of plastic wastes) [33]. PET can be recycled at four different levels: (i) primary recycling, involving the recycling (reutilization) of industrial scrap or waste in the form of raw materials to obtain the initial product quality; (ii) secondary recycling or physical recycling, involving contaminant removal, drying and melting processes of post-consumed PET products; (iii) tertiary recycling or chemical recycling, involving the depolymerization of PET chain into monomer units or larger oligomeric chains; and (iv) quaternary recycling involves the incineration of PET waste to produce energy [34]. In this review, we will focus on the tertiary and secondary recycling processes for synthesis of new raw materials for adhesive formulations and their performance in different binding systems.
2.3. Microencapsulation of Isocyanate Species for Eco-Innovative Adhesives Formulations
High quality, strong, and long-lasting adhesives used in the footwear, construction, automotive, and aerospace industries, such as those based on PU, polyurea (PUa), and polychloroprene (PCP), typically include highly reactive isocyanate species in their formulation, as cross-linking agents, to provide the required high strength ABJs. However, isocyanates’ high toxicity is a primary concern when applying a two-component formulation (2K) PU or PCP adhesive, and the current safety regulations that limit its use in the industry must be taken into account [35][36].
The ongoing need for minimizing the hazards associated with the various components, as well as product degradation is the driving force for the exploration of microencapsulation in the adhesives field. Indeed, microencapsulation (MCs) is a promising solution to minimize the risks related to the handling and storage of hazardous ingredients included in adhesive formulations. Dominating this field is an opportunity to innovation in order to address the challenge posed by current and future safety regulations.
The first work, reported in the literature, on the microencapsulation of isocyanate compounds in the liquid state dates from 2008, with the encapsulation of isophorone diisocyanate (IPDI) targeting an application for self-healing polymers [37]. Recently, this type of MCs has been optimized and tailored for other applications, regarding adhesives formulations. PUa/PU shell MCs containing high loadings of IPDI in the core were developed to enable the production of mono-component, eco-friendly, and safer adhesive formulations for the footwear industry [38]. MCs containing not only IPDI, but also commercial isocyanate oligomeric and prepolymeric species, have been successfully developed [38][39][40] for this particular application, but also for other applications, such as self-healing in epoxy resin matrices [41][42].
The developed MCs are envisaged to have the following characteristics: high core content, a long shelf-life, mechanical and chemical resistance, and be able to release all the encapsulated isocyanate at the moment of the ABJ´s preparation, triggered by mechanical and thermal stimuli.
The microencapsulation of isocyanate is typically achieved by an oil-in-water (O/W) micro-emulsion system combined with interfacial polymerization, which involves the addition of, at least, two reactants in a pair of immiscible liquids. One of the liquids is preferably an aqueous solution, forming the continuous phase (W phase) while the other, the dispersed phase, is composed by the isocyanate to encapsulate (O phase) in the presence of an emulsifier/surfactant. Both phases (water-based and oil-based solution) contain reactive species (OH groups and isocyanate (NCO) groups, respectively) which react together to form an initial thin PUa or PU polymeric shell. The polymerization is controlled mostly by diffusion, so the growth rate of the microcapsules (MCs) shell will decrease as the shell thickness increases [43].
The thermal and mechanical resistance of the MCs´ shell can be tailored by the addition of “latent” active hydrogen (H) sources that are not readily able to react with the isocyanate NCO groups. For instance, by addition of silanes (e.g., aminosilane and n-octyl triethoxysilane, n-OTES) in W phase, silanol Si-OH groups will be formed, which will react with NCO, forming urethane moieties. By condensation reactions (Si–O–Si linkages), additional improvement of the shell’s mechanical properties and hydrophobicity are expected [38][40].
Better encapsulation efficiency is achieved by using a more reactive isocyanate than the one to be encapsulated, which will act as shell forming material. For instance, a commercial type of oligomeric MDI with increased functionality, Ongronat® 2500 (Kazincbarcika, Hungary), with higher reactivity than IPDI, was employed as shell forming material [38]. Four different active H sources were tested, namely 3-(2-aminoethylamino) propyltrimethoxysilane (APTMS), tetraethyl orthosilicate, diethylenetriamine (DETA), and 3-isocyanatopropyltriethoxysilane (IPES), aiming at achieving a high encapsulation yield. The incorporation of a multifunctional isocyanate silane in the O phase, as “latent” active H source, led to the formation of impermeable PUa/PU-silica hybrid shell MCs (I MCs) with more than 60 wt.% of pure encapsulated IPDI [38].
The resulting MCs were incorporated in adhesive formulations and ABJs were prepared and compared with formulation without encapsulation of IPDI. The ones with encapsulated IPDI were found to exhibit the same peeling strength as the ones with non-encapsulated IPDI, which reveals the effective release and cross-linking ability of the encapsulated IPDI, in the form of a new generation of greener mono-component adhesives (Table 3).
Table 3. Peeling strength tests’ results [38].
|
Crosslinker Added to the OH Pre-Polymer |
Average Load per Unit Width of Bond |
Type of Failure Observed in the Peeling Strength Test |
|
None |
< 2 N/mm |
Adhesive, at the substrate/adhesive interface |
|
IPDI |
2.97 N/mm |
Cohesive, through the adhesive |
|
Microencapsulated IPDI (I MCs) |
2.99 N/mm |
Structural and cohesive rupture |
MCs encapsulating IPDI and MDI pre-polymer via O/W emulsion with Arabic gum as a prediluted emulsifier, for adhesive formulations purpose, were also obtained via an innovative process, consisting of a microfluidic device [39]. The main advantage of this emulsification method is the ability to produce a narrow MCs size distribution. The MCs obtained via this new method were found to exhibit an IPDI encapsulation efficiency, as high as the traditional (batch) method, however, the yield of MCs obtention is not as high as by the batch process.
Moreover, it should be noted that the encapsulation of oligomeric, or pre-polymeric isocyanates by a PU/PUa shell is potentially preferred, instead of the microencapsulation of monomeric isocyanates, since it brings higher reactivity in what regards the formation of a 3D polymeric structure in the ABJs, or in self-healing applications. However, the encapsulation of these species, including the commercially available ones, is scarcely found in the literature and frequently involves synthesis issues due to high viscosities and difficulty to control the high reactivities, unless an organic solvent is added to the isocyanate solution (O phase) [40].
The microencapsulation of a highly reactive, commercial oligomeric MDI, Ongronat® 2500, a medium viscosity liquid, solvent-free, with an increased functionality of 31 wt.% NCO, was recently reported [40], to be used as a crosslinker in PU-based adhesive formulations applications. In this work, the isocyanate species were encapsulated by a PU/PUa and a PU/PUa-silica hybrid shell, using a one-pot and straightforward approach, consisting of an O/W microemulsion system combined with an in situ polymerization at the interface of the O/W phases. The morphology of the MCs was studied along the synthesis, and a free-flowing powder was achieved at the end (Figure 2), ready to be incorporated into adhesive formulations.

Figure 2. MCs morphology evolution during synthesis: (a) microemulsion before addition of the active H source, (b) shell formation after addition of the active H source, (c) free flowing powder consisting of the MCs after synthesis (scale 1:1). [40] Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer, Journal of Materials Science, “The role played by different active hydrogen sources in the microencapsulation of a commercial oligomeric diisocyanate”, by Loureiro, et al. (2020).
The role played by a variety of amine based active H sources, namely ethylenediamine (EDA), APTMS and branched poly(ethyleneimine) (PEI), and the silane n-OTES, that simultaneously acts as a “latent” active H source and a hydrophobic agent was studied in terms of encapsulation efficiency, shelf-life, shell´s thermal stability and chemical structure for new potential adhesive formulations. The best overall performance was achieved for the combination of the high NH (amino) functionality PEI and the silane n-OTES, as active H sources (nOTES_PEI_MCs) with a loss of only 34% of the encapsulated isocyanate Ongronat® 2500 during a period of seven months, in contrast with the 77.4% lost by the MCs synthesized without any additional active H source. They were found to be robust and provide an effective barrier against air’ moisture, which is critical for their future application as crosslinkers for eco-innovative 1K adhesive formulations [40].
More recently, MCs containing isocyanate made of a biodegradable shell have been reported, which further contributes to eco-innovative adhesives [42]. In this pioneering study, a biodegradable polymer, polycaprolactone (PCL), was used as shell material for the encapsulation of isocyanate species, which brings a further advantage, related with its thermal response in the temperature range of the adhesive joints manufacture. The obtained ABJs incorporating the MCs were found to exhibit the same peeling strength as the sample with non-encapsulated isocyanate, which reveals the effective IPDI release from the MCs core and its further reaction with the OH-prepolymer leading to a final crosslinked adhesive.
Thus, we highly believe that the encapsulation of high loadings of reactive isocyanate species enables their use as cross-linking agents in safer, eco-innovative, 1K and high-performing adhesive formulations, which can be further extended to other applications, such as self-healing approaches and smart materials.
2.4. Final Considerations on the State of the Art of Eco-Friendly Structural Adhesives
The strive for monomers derived from non-virgin petrochemical and bio-based raw materials, that are scalable, abundant and truly sustainable is a reality, and it is stimulating R&D activities at the global level and promoting the creation of new “green” businesses opportunities. Vegetable oils, wood derivates (sustainable biomass), polysaccharides, and recycled plastics are alternative feedstocks that are gaining importance in this field. However, mechanical properties of the adhesive performance are often compromised when moving away from conventional, well established petrochemical-derived plastics. Strategies, such as the incorporation of nanoparticles, or the microencapsulation of hazardous, but efficient cross-linkers are potential solutions to enable eco-friendly structural adhesives.
Finally, it should be stressed that a sustainability assessment combining the technical, economic/operational, environmental, and social dimensions should be implemented in what regards eco-friendly adhesives. However, such assessment, when applied to emergent materials and technologies, represents a significant challenge because there is a lack of proper indicators, lack of information, and contradictory information is common. This is the case of bio-derived adhesives, which might originate from nature-based materials harvested in a myriad of ways in several regions of the globe. Additionally, optimization of production systems is required to be technically and environmentally competitive.
Bio-adhesives derived from different available renewable biopolymers such as protein (soy) and lignin (Kraft and Organosolv), as well as tannin, were employed in a cradle-to-gate life cycle assessment or analysis (LCA) [44]. Other studies that compare bio- with petro-chemical adhesives, or potential greener processes, are found in [45][46][47][48][49]. A systematic literature review linking the sustainable assessment approach and biocomposite materials has been carried out [50]. The authors claim that life cycle sustainability assessment, integrating ecological, financial and social parameters, life cycle engineering, integrating the technical and functional aspects, and eco-design play a crucial role in encouraging life cycle thinking in decision-making for stakeholders, public authorities, and consumers, in these types of materials.
3. Surface Treatments to Enable Emergent Technologies
The creation of defined surface properties is the main goal to obtain reproducible bonding results—this is the decisive challenge for the effort in selecting a suitable pre-treatment [51]. Improving the adhesion between the substrate and the adhesive often requires modifying different surface properties that are known to influence adhesion. The modified properties can be either physical (mechanical), chemical, or, ideally, both. Physical modifications increase the surface roughness of the substrate to provide better wetting of the adhesive and a larger surface area. This contributes on a macroscopic level, as mechanical forces between the substrate and the adhesive also promotes adhesion. These types of interactions are introduced by changes in the surface morphology. According to the mechanical theory, adhesion occurs as a result of the adhesive penetrating into cavities, voids, or pores on the surface [52]. This theory is supported by experimental results showing an increase in joint strengths after mechanical roughening of the surface using grit blasting or mechanical abrasion [53]. Further, even greater strengths are achieved by the presence of an open porous structure formed by anodizing aluminium in acid electrolytes that leads to micro- and nano- surface roughness. This phenomenon is referred to as “mechanical interlocking”. The interface is then seen as a composite layer that enables better stress distribution and arrests the propagation of cracks during mechanical stress, see Figure 3a. Too much roughness, however, also has its disadvantages as it can lead to incomplete initial wetting of the surface and to the creation of voids that can act as failure initiation points due to stress concentrations [52]. Chemical modification of the surface improves its ability of the surface to form chemical bonds with the polymeric adhesive providing functional groups (e.g., silanes, hydroxides) that are compatible with the chosen adhesive and/or by increasing the density of these functional groups (Figure 3b). Acid etching, anodizing, and the use of coupling agents are the most commonly used methods to functionalize metal substrate surfaces. In practice, chemical and mechanical contributions are interrelated. After all, an increase in the surface roughness also leads to an increase in the surface area available for molecular and atomic interactions.
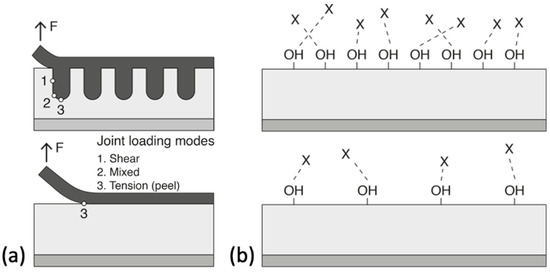
Figure 3. Schematic illustrations of the (a) mechanical advantage provided by surface treatments that increase the surface roughness and bonding area and (b) the effect of hydroxyl density on interfacial bonding with adhesive (represented by X). Reprinted with adjustments from [54] http://creativecommons.org/licenses/by/4.0/.
There is a variety of adhesion theories, such as mechanical interlocking, electrostatic adhesion, wettability, surface energy and thermodynamic adhesion, diffusion, weak boundary layer, and acid-base and covalent bonding theory, which can be applied for numerous substrate-adherent combinations, including natural materials, such as wood substrates.
Various processes are available to introduce both types of modifications, physical and chemical, which must be selected depending on the substrate, the adhesive, and the application requirements.
Depending on the given conditions, three main groups of surface treatment must be considered:
-
Surface preparation [55], including cleaning [55] (removing dust, rust, contaminants like oils and grease), and geometrical adjustment (deburring);
-
Surface pre-treatment, which can be physical-mechanical, physical, and chemical;
-
Surface post-treatment, which contains the application of primers and the climatization.
4. Disassembling Strategies for Maintenance and End-of-Life of Structural Adhesives
Today, many commercial adhesives are developed to reach the highest possible adhesive and cohesive strength and to last “forever.” As tempting as “forever” sounds, it is not desirable in many applications (e.g., products with high complexity or cost) since maintenance, repair works, or upgrades must be done from time to time [56].
Further, recycling at the end of the product’s life cycle must be considered. Environmental requirements, such as the EU End-of-Life Vehicle (ELV) Directive, demand that all cars must be recyclable at a minimum of 95% of the vehicle mass [57]. In all these cases, there is a need for adhesive technologies, which allow debonding of the surfaces. By debonding, or disassembly, we mean the breaking of the polymer chains into shorter sub-units and monomers, to aid maintenance, or recycling operations. Disassembly may be triggered at an appropriate time, either by a chemical or physical-assisted approaches.
With this in mind, Figure 4 gives an overview of the materials flows within the life cycle of an adhesive-bonded joint, recalling that formulation components and adhesive structures should be designed with materials recovery in mind [58], to promote the reuse, repairing, and recycling of implicated materials.
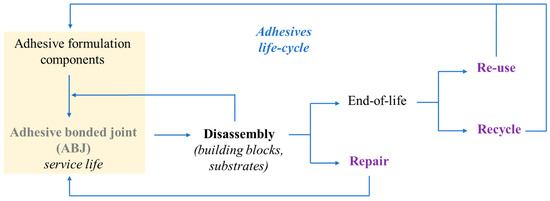
Figure 4. A life-cycle diagram, showing adhesive materials flows. Adapted from [58] “Overview of disbonding technologies for adhesive bonded joints”, A. Hutchinson, Y. Liu, et al. Journal of Adhesion, Vol 93, Pages No. 737–755, Copyright (2016), with permission from Taylor & Francis Ltd., http://www.tandfonline.com.
There are many concepts and patents published [59][60][61][62][63][64][65][66][67][68][69][70][71][72] that deal with debonding surfaces, but only a few solutions are available on the market. All of them have their advantages, drawbacks, and limitations. In the following, we give an overview of the existing technologies concerning the debonding of structural adhesive bonds.
This entry is adapted from the peer-reviewed paper 10.3390/ma13245590
References
- Shadlou, S.; Ahmadi-Moghadam, B.; Taheri, F. Nano-Enhanced Adhesives. Rev. Adhes. Adhes. 2014, 2, 371–412.
- Kaybal, H.B.; Ulus, H.; Avcı, A. Influence of Nano-CaCO3 Particles on Shear Strength of Epoxy Resin Adhesives. Int. J. Eng. Res. Dev. 2017, 9, 29–35.
- Science to Enable Sustainable Plastics—A White Paper from the 8th Chemical Sciences and Society Summit (CS3). 2020. Available online: (accessed on 28 June 2020).
- Heinrich, L.A. Future opportunities for bio-based adhesives-advantages beyond renewability. Green Chem. 2019, 21, 1866–1888.
- Jeevi, G.; Nayak, S.K.; Kader, M.A. Review on adhesive joints and their application in hybrid composite structures. J. Adhes. Sci. Technol. 2019, 33, 1497–1520.
- Banea, M.D.; Da Silva, L.F.M. Adhesively bonded joints in composite materials: An overview. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2009, 223, 1–18.
- Budhe, S.; Banea, M.D.; De Barros, S. Bonded repair of composite structures in aerospace application: A review on environmental issues. Appl. Adhes. Sci. 2018, 6, 3.
- Hemmilä, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of sustainable bio-adhesives for engineered wood panels—A Review. RSC Adv. 2017, 7, 38604–38630.
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Bio-Renewable Sources for Synthesis of Eco-Friendly Polyurethane Adhesives—Review. Open J. Polym. Chem. 2017, 7, 57–75.
- Magalhães, S.; Alves, L.; Medronho, B.; Fonseca, A.C.; Romano, A.; Coelho, J.; Norgren, M. Brief overview on bio-based adhesives and sealants. Polymers 2019, 11, 1685.
- Messler, R.W., Jr. Joining of Materials and Structures—From Pragmatic Process to Enabling Technology; Elsevier Butterworth-Heinemann: Oxford, UK, 2004.
- Wahab, M.A. JOINING COMPOSITES with ADHESIVES Theory and Applications; DEStech Publications, Inc.: Lancaster, PA, USA, 2016; Available online: (accessed on 15 June 2020).
- Ebnesajjad, S. Handbook of Adhesives and Surface Preparation: Technology, Applications and Manufacturing; Elsevier: Oxford, UK, 2011.
- Baudrit, J.R.V.; Camacho, N.C.; Urena, Y.C. Basis and Applications of Silicon Reinforced Adhesives. Org. Med. Chem. Int. J. 2018, 5, 1–12.
- Adhesive Bonding of Composites. 2020. Available online: (accessed on 19 March 2020).
- Cârciumaru, M.; Ion, R.M.; Niţu, E.C.; Ştefânescu, R. New evidence of adhesive as hafting material on Middle and Upper Palaeolithic artefacts from Gura Cheii-Râşnov Cave (Romania). J. Archaeol. Sci. 2012, 39, 1942–1950.
- Karpowicz, A. Ottoman Turkish Bows, Manufacture and Design; The Lyons Press: Guilford, NC, USA, 1992.
- Packham, D.E. Adhesive technology and sustainability. Int. J. Adhes. Adhes. 2009, 29, 248–252.
- Isa, Y.M.; Ganda, E.T. Bio-oil as a potential source of petroleum range fuels. Renew. Sustain. Energy Rev. 2018, 81, 69–75.
- Sharma, V.; Getahun, T.; Verma, M.; Villa, A.; Gupta, N. Carbon based catalysts for the hydrodeoxygenation of lignin and related molecules: A powerful tool for the generation of non-petroleum chemical products including hydrocarbons. Renew. Sustain. Energy Rev. 2020, 133, 110280.
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.C.; Xu, C. Global lignin supply overview and kraft lignin potential as an alternative for petroleum-based polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768.
- Vnučec, D.; Kutnar, A.; Goršek, A. Soy-based adhesives for wood-bonding—A review. J. Adhes. Sci. Technol. 2017, 31, 910–931.
- Siracusa, V. Microbial degradation of synthetic biopolymers waste. Polymers 2019, 11, 1066.
- Ashter, S.A. Mechanisms of Polymer Degradation. In Introduction to Bioplastics Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 31–59.
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344.
- Ciardiello, R. Mechanical characterization and separation tests of a thermoplastic reinforced adhesive used for automotive applications. Procedia Struct. Integr. 2019, 24, 155–166.
- Onusseit, H. The influence of adhesives on recycling. Resour. Conserv. Recycl. 2006, 46, 168–181.
- Hahladakis, J.N.; Iacovidou, E. An overview of the challenges and trade-offs in closing the loop of post-consumer plastic waste (PCPW): Focus on recycling. J. Hazard. Mater. 2019, 380, 120887.
- G.M.R. Plastics Europe, Conversio Market & Strategy GmbH, Plastics—The Facts 2019. 2019. Available online: (accessed on 28 June 2020).
- Alhareb, A.O.; Akil, H.M.; Ahmad, Z.A. Impact strength, fracture toughness and hardness improvement of PMMA denture base through addition of nitrile rubber/ceramic fillers. Saudi J. Dent. Res. 2017, 8, 26–34.
- Ragaert, K.; Delva, L.; van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58.
- European Strategy for Plastics in a Circular Economy. 2019. Available online: (accessed on 16 July 2020).
- Jasiukaitytė-Grojzdek, E.; Kunaver, M.; Kukanja, D.; Moderc, D. Renewable (waste) material based polyesters as plasticizers for adhesives. Int. J. Adhes. Adhes. 2013, 46, 56–61.
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64.
- Pizzi, K.L.; Mittal, A. Handbook of Adhesive Technology; CRC Press: New York, NY, USA, 2017.
- Registry of Restriction Intentions Until Outcome. 2019. Available online: (accessed on 25 June 2020).
- Yang, J.; Keller, M.W.; Moore, J.S.; White, S.R.; Sottos, N.R. Microencapsulation of Isocyanates for Self-Healing Polymers. Macromolecules 2008, 41, 9650–9655.
- Attaei, M.; Loureiro, M.; Vale, M.d.; Condeço, J.; Pinho, I.; Bordado, J.; Marques, A. Isophorone Diisocyanate (IPDI) Microencapsulation for Mono-Component Adhesives: Effect of the Active H and NCO Sources. Polymers 2018, 10, 825.
- Costa, M.; Dias, J.P.; Pinho, I.; Loureiro, M.V.; Marques, A.C.; Simoes, R. Development of a Microfluidic Device to Encapsulate Isocyanate for Autoreactive and Ecological Adhesives. IOP Conf. Ser. Mater. Sci. Eng. 2019, 520, 012007.
- Loureiro, M.V.; Attaei, M.; Rocha, S.; Vale, M.; Bordado, J.C.; Simões, R.; Pinho, I.; Marques, A.C. The role played by different active hydrogen sources in the microencapsulation of a commercial oligomeric diisocyanate. J. Mater. Sci. 2019, 55, 4607–4623.
- Attaei, M.; Vale, M.; Shakoor, A.; Kahraman, R.; Montemor, M.F.; Marques, A.C. Hybrid shell microcapsules containing isophorone diisocyanate with high thermal and chemical stability for autonomous self-healing of epoxy coatings. J. Appl. Polym. Sci. 2019, 137, 48751.
- Attaei, M.; Calado, L.M.; Taryba, M.G.; Morozov, Y.; Shakoor, R.A.; Kahraman, R.; Marques, A.C.; Montemor, M.F. Autonomous self-healing in epoxy coatings provided by high efficiency isophorone diisocyanate (IPDI) microcapsules for protection of carbon steel. Prog. Org. Coat. 2020, 139, 105445.
- Duan, B. Microencapsulation via In Situ Polymerization. In Handbook of Encapsulation and Controlled Release; CRC Press: Boca Raton, FL, USA, 2015; pp. 307–314.
- Arias, A.; González-García, S.; González-Rodríguez, S.; Feijoo, G.; Moreira, M.T. Cradle-to-gate Life Cycle Assessment of bio-adhesives for the wood panel industry. A comparison with petrochemical alternatives. Sci. Total. Environ. 2020, 738, 140357.
- McDevitt, J.E.; Grigsby, W.J. Life Cycle Assessment of Bio- and Petro-Chemical Adhesives Used in Fiberboard Production. J. Polym. Environ. 2014, 22, 537–544.
- Ferrari, A.M.; Spinelli, R.; Gamberini, R.; Neri, P.; Pini, M.; Rimini, B. Comparative life cycle assessment of innovative and traditional adhesives for the laying of ceramic tiles. Int. J. Oper. Quant. Manag. 2014, 20, 227–241.
- la Rosa, A.D.; Ursan, G.A.; Aradoaei, M.; Ursan, M.; Schreiner, C. Life Cycle Assessment of Microwave Activated Hot-Melt Adhesives. In Proceedings of the EPE 2018—2018 10th International Conference and Exposition on Electrical and Power Engineering, Iaşi, Romania, 18–19 October 2018; pp. 658–661.
- Yang, M.; Rosentrater, K.A. Cradle-to-gate life cycle assessment of structural bio-adhesives derived from glycerol. Int. J. Life Cycle Assess 2020.
- Yang, M.; Rosentrater, K.A. Life Cycle Assessment and Techno-Economic Analysis of Pressure Sensitive Bio-Adhesive Production. Energies 2019, 12, 4502.
- Rodriguez, L.J.; Peças, P.; Carvalho, H.; Orrego, C.E. A literature review on life cycle tools fostering holistic sustainability assessment: An application in biocomposite materials. J. Environ. Manag. 2020, 262, 110308.
- Habenicht, G. Applied Adhesive Bonding; WILEY VCH: Weinheim, German, 2009.
- Packham, D.E. Theories of fundamental adhesion. In Handbook of Adhesion Technology, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2018.
- Rider, A.N.; Olsson-Jacques, C.L.; Arnott, D.R. Influence of adherend surface preparation on bond durability. Surf. Interface Anal. 1999, 27, 1055–1063.
- Abrahami, S.T.; de Kok, J.M.M.; Gudla, V.C.; Ambat, R.; Terryn, H.; Mol, J.M.C. Interface strength and degradation of adhesively bonded porous aluminum oxides. NPJ Mater. Degrad. 2017, 1, 8.
- ISO 17212. Structural Adhesives—Guidelines for the Surface Preparation of Metals and Plastics Prior to Adhesive Bonding. 2012. Available online: (accessed on 18 June 2020).
- Banea, M.D. Debonding on Demand of Adhesively Bonded Joints: A Critical Review. Rev. Adhes. Adhes. 2019, 7, 33–50.
- EC. Directive 200053EC of the European Parliament and of the Council on End-of Life vehicles. Off. J. Eur. Communities L 2000, 269, 1–15.
- Hutchinson, A.; Liu, Y.; Lu, Y. Overview of disbonding technologies for adhesive bonded joints. J. Adhes. 2017, 93, 737–755.
- Heucher, R.; Kopannia, S.; Mcardle, C.; Stuve, M.; Kolbe, J. Electrically Divisible Polyamide Adhesive. KR20140133606A, 12 March 2013.
- Kristen, C. Process for Adhesive Separation of Bonded Joints. US20050039848A1, 5 August 2008.
- Markle, I.R.A.; Phyllis, L.; George, E.; Elhard, J.D.; Bigg, D.M. Thermally Reversible Isocyanate-Based Polymers. U.S. Patent 5470945A, 28 November 1995.
- Alcorta, J.; Papon, E.; Villenave, J.-J. Destructuring Agent for an Adhesive Composition, and Glue and Primer Forming Said Composition. WO2005028583A1, 31 May 2005.
- Licari, J.J.; Bakhit, G.G. Reworkable Epoxy Die-Attach Adhesive. U.S. Patent US5002818A, 26 March 1991.
- Foulc, M.; Bergara, T.; Olive, M. Assembly of Two Substrates Bonded by a Rigid Polymer, and Methods for Assembly and Dismantling by Means of Migration of Said Bonded Assembly. U.S. Patent 20120258315A1, 11 October 2012.
- Becher, P.; Flegel, H.; Hermann, M.; Kurzmann, P.; Bauer, J.; Bauer, M.; Krüger, H.; Neumann-Rodekrich, J.; Schneider, J.; Hirthhammer, M. Adhesive System for Form Reversible Glued Joints. Eur. Pat. EP1115770B1, 10 November 2004.
- Gilbert, M.D. Electrically Disbonding Materials. U.S. Patent 7968188B2, 28 June 2011.
- Gilbert, M.D. Electrically Disbonding Adhesive Compositions and Related Methods. U.S. Patent 7465492B2, 16 December 2008.
- Friese, C.; Unger, L.; Kirsten, C.; Huver, T.; Ferencz, A. Lösbare Klebeverbindungen. Eur. Pat. EP1111020A2, 27 June 2001.
- Morehouse, D.S.; Tetreault, R.J. Expansible Thermoplastic Polymer Particles Containing Volatile Fluid Foaming Agent and Method of Foaming the Same. U.S. Patent 3615972A, 28 April 1967.
- Bain, P.; Giovanni, M. Method and apparatus for bonding and debonding adhesive interface surfaces. WO2004087826A3, 13 January 2011.
- Aubert, J. Method of Making Thermally Removable Adhesives. U.S. Patent 20030116272A1, 26 June 2003.
- Afzali-Ardakani, A.; Buchwalter, S.; Gelorme, J.; Kosbar, L.; Newman, B.; Pompeo, F. Cleavable Diepoxide for Removable Epoxy Compositions. U.S. Patent 5560934A, 1 October 1996.
