Sarcopenia is a geriatric syndrome characterized by the progressive degeneration of muscle mass and function. It is associated with cognitive impairment, defined as a decline in cognitive domains such as language, memory, reasoning, social cognition, planning, making decisions, and solving problems. Several studies have shown that skeletal muscle can regulate brain functions, including mood, learning, locomotor activity, and neuronal injury protection, showing the existence of muscle-brain crosstalk.
- myokines
- exerkines
- skeletal muscle
- sarcopenic
1. Sarcopenia
Sarcopenia is a common condition in older individuals, characterized by progressive muscle mass and function degeneration. It is associated with severe complications, including falls, functional decline, frailty, and mortality [1][2]. The prevalence of sarcopenia varies from 9.9% to 40.4%, depending on its definition [1]. Nowadays, there is no consensus on defining the cut-off points, making sarcopenia diagnosis challenging.
The pathogenesis of sarcopenia remains poor clear and involves an interplay between sedentary lifestyle, aging, obesity, inflammation, and oxidative stress that affect muscle mass and function [2].
A sedentary lifestyle, defined as activities that do not increase energy expenditure, impacts muscle mass and metabolism. Indeed, only seven days of decubitus resulted in a loss of muscle mass, and a prolonged period, 90–120 days, reduced 30% of the muscle volume [3][4]. Studies conducted on old immobilized animals have examined the effects of bed rest on skeletal muscle metabolism, demonstrating a disruption in the balance between protein synthesis and degradation in favor of catabolism [3][4].
Interestingly, aging alters both the homeostasis of skeletal muscle, compromising the equilibrium between cell regeneration and differentiation [5] and the rate of protein synthesis and degradation [6]. It is associated with reducing skeletal muscle stem cells (satellite cells) in type II fiber. Major pathways associated with changes in satellite cells during aging include Notch and Wnt signaling; the first is associated with proliferation while the second is differentiation of muscle cells [7]. Studies demonstrated that the expression of Notch signaling decreased with age during aging [8]. Wnt canonical pathway switched to the not canonical pathway resulting in the inability of satellite cells to self-renewal [9]. However, the hypothesis that loss of satellite cell activity is the cause of sarcopenia has been confuted. In male sedentary mice, the depletion of satellite cells, resulting in impaired muscle regeneration, did not contribute to muscle size or fiber type composition, despite low regenerative capacity, but contributed to age-related muscle fibrosis [10].
With advancing age, the intake of amino acids is inadequate, resulting in a decreased protein synthesis rate and the proteolysis system's inability (ubiquitination and lysosomal degradation) to remove oxidized proteins, inducing a progressive decline in skeletal muscle mass function [6][11].
Pathogenic inter-relationship between adipose tissue and muscle is also crucial in sarcopenia and contributes to functional and physiological impairment. Obesity is characterized by increased production of fatty acids (FAs) that are not only stored in adipose tissue (AT) but can outflow and accumulate ectopically in skeletal muscle [12]. FAs, in the form of triglycerides (TG), diacylglycerols (DAG), and ceramides, accumulate both in intermuscular adipose tissue (IMAT) as in intramyocellular lipids (IMCLs), inducing impaired single-fiber contractility via mitochondrial dysfunction, impaired β-oxidation of FAs, and increased reactive oxygen species (ROS) production, leading to lipotoxicity and insulin resistance (IR) [13][14].
These events' primary outcome is muscle fiber insufficiency with a decline in muscle mass and function [15]. Indeed, IMCLs attract immune cells, such as M1-type macrophages, mast cells, Th1, Th17, and other cells, that produce an array of pro-inflammatory cytokines [16][17][18][19]. Activated adipocytes produce pro-inflammatory adipokines, like leptin, osteopontin, chemerin, and a lower expression of SIRT1 in the subcutaneous abdominal fat [20], creating a pro-inflammatory vicious circle providing local and systemic, chronic low-grade inflammation [21][22], which is also related to glucose metabolism derangement [23]. Furthermore, this unfavorable adipokines/cytokine profile increases IR and contributes to ectopic fat distribution [24].
2. Sarcopenia as a Risk Factor for Cognitive Decline
In the literature, it is well documented that sarcopenia increases the risk of cognitive decline [25]. Despite the contradictory results that could be due to different criteria and cut-off points to assess used sarcopenia components [26], a recent systemic review and meta-analysis demonstrated that the association between sarcopenia and cognitive impairment was independent of the study population, sarcopenia definition, and cognitive impairment degree (odds ratio 2.2, 95% CI 1.2–4.2) [27].
In particular, a cross-sectional study based on 3025 women aged 75 years and older demonstrated an association between muscle strength, a central component of sarcopenia, and cognitive function. Lower handgrip (HGS), used to measure muscle strength, was associated with cognitive impairment, measured by a short portable mental status questionnaire (SPMSQ) (OR 1.81 and 95% confidence interval: 1.33–2.46) [28][29]. Which cognitive domains are affected by muscle strength are poorly described. A cross-sectional study, conducted on 1799 participants aged more than 60 years old, demonstrated a higher digit symbol substitution test (DSST) score, used to measure visuospatial and motor speed was more significant in higher quadriceps strength groups indicating that muscle strength was associated with frontal lobe executive functions [29]. Another study of 555 participants, aged 85 years at baseline, suggested that HGS was associated with processing speed and memory function [30].
Even muscle mass is considered a predictor of cognitive decline, the link between muscle mass and cognitive impairment is not consistently documented [26].
Although the exact mechanisms involved have not yet been defined, risk factors may partially explain the association between cognitive decline and sarcopenia. Direct cross-talk between muscle and brain, mediated by exercise-induced myokines release, has been demonstrated [31][32]. Physical activity restores and maintains cognitive functions and metabolism [33][34] and ameliorates the process of neurological diseases [35], inducing muscle cells, metabolically active, to produce and release myokines. It was proposed that all factors released in response to exercise should be termed "exerkines" [36].
3. Role of Physical Exercise in Muscle and Brain Cross-Talk
Physical activity is a non-pharmacological intervention that ameliorates brain function [37]. It has been reported that exercise increases the volume and intensifies the prefrontal cortex's function, hippocampus, which are neuronal regions related to memory and cognition [38][39][40][41]. Studies conducted on people with AD, the most common form of dementia, have demonstrated that exercise can improve cognitive and physical function [42]. Moreover, activity was associated with a 30–40% reduction in the risk of developing AD than physically inactive individuals [43].
A longitudinal observational study demonstrated an association between physical activity and a lower likelihood of cognitive decline (RR 0.65, 95% CI 0.55–0.76) [44]. Similar results were obtained from another study that demonstrated that the group with cognitive impairment had more deficient performance gait speed test than the control group [45]. The exercise-induced improvement in cognitive function was also demonstrated in older adults. A meta-analytic study examined aerobic fitness effects on cognitive vitality of healthy but sedentary older adults. The study has indicated that physical activity impacts positively on cognition [46].
Physical exercise mediates the beneficial effects promoting cerebral angiogenesis, increasing neurogenesis and plasticity of the hippocampus, increasing cerebral blood flow, diminishing blood-brain barrier (BBB) permeability and function [47], and enhancing oxygen-rich blood delivery to the brain [48][49][50][51].
In skeletal muscle, physical exercise activates compensatory and adaptive mechanisms to obtain energy that can be reached via metabolic regulation or changes in gene expression [52]. Exercise regulates myokines' expression, contributing to autocrine regulation of metabolism in the muscle and paracrine/endocrine regulation of other adjacent/remote organs [37]. Studies conducted on exercise showed that physical activity, increasing circulating levels of myokines in the bloodstream, exert beneficial effects on the brain. The myokines regulate brain functions, including mood, learning, locomotor activity, and protecting neuronal injury in animal or in vitro models [36][37][50].
So, altered synthesis and production of myokines due to physical inactivity may be associated with adverse implications in the brain, such as cognitive impairment and neurogenerative events [53], showing that muscle may influence the brain's health (Figure 1).
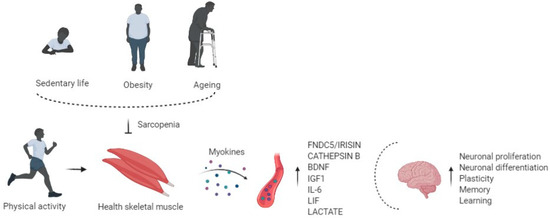
Figure 1. Physical activity enhances circulating levels of myokines in the bloodstream, affects the brain regulating neuronal proliferation and differentiation, plasticity, memory, and learning. Risk factors of sarcopenia, such as physical inactivity, obesity, and aging, alter the myokines' production and release, impairing cognitive function.
This entry is adapted from the peer-reviewed paper 10.3390/life11020173
References
- Kaeberlein, M.; Rabinovitch, P.S.; Martin, G.M. Healthy aging: The ultimate preventative medicine. Science 2015, 350, 1191–1193.
- Molino, S.; Dossena, M.; Buonocore, D.; Verri, M. Sarcopenic obesity: An appraisal of the current status of knowledge and management in elderly people. J. Nutr. Health Aging 2015, 20, 780–788.
- Alkner, B.A.; Tesch, P.A. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur. J. Appl. Physiol. 2004, 93, 294–305.
- Shackelford, L.C.; Leblanc, A.D.; Driscoll, T.B.; Evans, H.J.; Rianon, N.J.; Smith, S.M.; Spector, E.; Feeback, D.L.; Lai, D. Resistance exercise as a countermeasure to disuse-induced bone loss. J. Appl. Physiol. 2004, 97, 119–129.
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646.
- Murton, A.J.; Marimuthu, K.; Mallinson, J.E.; Selby, A.L.; Smith, K.; Rennie, M.J.; Greenhaff, P.L. Obesity Appears to Be Associated With Altered Muscle Protein Synthetic and Breakdown Responses to Increased Nutrient Delivery in Older Men, but Not Reduced Muscle Mass or Contractile Function. Diabetes 2015, 64, 3160–3171.
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt Signaling During Aging Alters Muscle Stem Cell Fate and Increases Fibrosis. Science 2007, 317, 807–810.
- Carey, K.A.; Farnfield, M.M.; Tarquinio, S.D.; Cameron-Smith, D. Impaired Expression of Notch Signaling Genes in Aged Human Skeletal Muscle. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2007, 62, 9–17.
- Florian, M.C.; Nattamai, K.J.; Dörr, K.; Marka, G.; Überle, B.; Vas, V.; Eckl, C.; Andrä, I.; Schiemann, M.; Oostendorp, R.A.J.; et al. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nat. Cell Biol. 2013, 503, 392–396.
- Fry, C.S.; Lee, J.D.; Mula, J.; Kirby, T.J.; Jackson, J.R.; Liu, F.; Yang, L.; Mendias, C.L.; Dupont-Versteegden, E.E.; McCarthy, J.J.; et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 2015, 21, 76–80.
- Marcell, T.J. Review Article: Sarcopenia: Causes, Consequences, and Preventions. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2003, 58, M911–M916.
- Rutkowski, J.M.; Stern, J.H.; Scherer, P.E. The cell biology of fat expansion. J. Cell Biol. 2015, 208, 501–512.
- Kob, R.; Bollheimer, L.C.; Bertsch, T.; Fellner, C.; Djukic, M.; Sieber, C.C.; Fischer, B.E. Sarcopenic obesity: Molecular clues to a better understanding of its pathogenesis? Biogerontology 2014, 16, 15–29.
- Stinkens, R.; Goossens, G.H.; Jocken, J.W.E.; Blaak, EE. Targeting fatty acid metabolism to improve glucose metabolism. Obes. Rev. 2015, 16, 715–757.
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221.
- Rivas, D.A.; McDonald, D.J.; Rice, N.P.; Haran, P.H.; Dolnikowski, G.G.; Fielding, R.A. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am. J. Physiol. Integr. Comp. Physiol. 2016, 310, R561–R569.
- Apostolopoulos, V.; De Courten, M.P.J.; Stojanovska, L.; Blatch, G.L.; Tangalakis, K.; De Courten, B.; de Courten, M.P.M.P.J. The complex immunological and inflammatory network of adipose tissue in obesity. Mol. Nutr. Food Res. 2015, 60, 43–57.
- Exley, M.A.; Hand, L.; O’Shea, D.; Lynch, L. Interplay between the immune system and adipose tissue in obesity. J. Endocrinol. 2014, 223, R41–R48.
- Tateya, S.; Kim, F.; Tamori, Y. Recent advances in obesity-induced inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 93.
- Sardu, C.; Pieretti, G.; D’Onofrio, N.; Ciccarelli, F.; Paolisso, P.; Passavanti, M.B.; Marfella, R.; Cioffi, M.; Mone, P.; Dalise, A.M.; et al. Inflammatory Cytokines and SIRT1 Levels in Subcutaneous Abdominal Fat: Relationship With Cardiac Performance in Overweight Pre-diabetics Patients. Front. Physiol. 2018, 9, 1030.
- Raschke, S.; Eckel, J. Adipo-Myokines: Two Sides of the Same Coin—Mediators of Inflammation and Mediators of Exercise. Mediat. Inflamm. 2013, 2013, 1–16.
- Rodríguez, A.; Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Frühbeck, G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Metab. 2015, 309, E691–E714.
- Paolisso, P.; Foà, A.; Bergamaschi, L.; Donati, F.; Fabrizio, M.; Chiti, C.; Angeli, F.; Toniolo, S.; Stefanizzi, A.; Armillotta, M.; et al. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovasc. Diabetol. 2021, 20, 1–11.
- Kim, T.N.; Park, M.S.; Lim, K.I.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Choi, H.; Baik, S.H.; et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: The Korean Sarcopenic Obesity Study. Clin. Endocrinol. 2013, 78, 525–532.
- Cipolli, G.C.; Yassuda, M.S.; Aprahamian, I. Sarcopenia Is Associated with Cognitive Impairment in Older Adults: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2019, 23, 525–531.
- Sui, S.X.; Williams, L.J.; Holloway-Kew, K.L.; Hyde, N.K.; Pasco, J.A. Skeletal Muscle Health and Cognitive Function: A Narrative Review. Int. J. Mol. Sci. 2020, 22, 255.
- Peng, T.-C.; Chen, W.-L.; Wu, L.-W.; Chang, Y.-W.; Kao, T.-W. Sarcopenia and cognitive impairment: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2695–2701.
- Van Kan, G.A.; Cesari, M.; Gillette-Guyonnet, S.; Dupuy, C.; Nourhashémi, F.; Schott, A.-M.; Beauchet, O.; Annweiler, C.; Vellas, B.; Rolland, Y. Sarcopenia and cognitive impairment in elderly women: Results from the EPIDOS cohort. Age Ageing 2012, 42, 196–202.
- Chen, W.-L.; Peng, T.-C.; Sun, Y.-S.; Yang, H.-F.; Liaw, F.-Y.; Wu, L.-W.; Chang, Y.-W.; Kao, T.-W. Examining the Association Between Quadriceps Strength and Cognitive Performance in the Elderly. Medicine 2015, 94, e1335.
- Taekema, D.G.; Ling, C.H.Y.; Kurrle, S.E.; Cameron, I.D.; Meskers, C.G.M.; Blauw, G.J.; Westendorp, R.G.J.; De Craen, A.J.M.; Maier, A.B. Temporal relationship between handgrip strength and cognitive performance in oldest old people. Age Ageing 2012, 41, 506–512.
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609.
- Chen, W.; Wang, L.; You, W.; Shan, T. Myokines mediate the cross talk between skeletal muscle and other organs. J. Cell. Physiol. 2021, 236, 2393–2412.
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472.
- Mattson, M.P. Evolutionary aspects of human exercise—Born to run purposefully. Ageing Res. Rev. 2012, 11, 347–352.
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal Muscle PGC-1α1 Modulates Kynurenine Metabolism and Mediates Resilience to Stress-Induced Depression. Cell 2014, 159, 33–45.
- Pedersen, B.K. Physical activity and muscle-brain cross-talk. Nat. Rev. Endocrinol. 2019, 15, 383–392.
- Kim, S.; Choi, J.-Y.; Moon, S.; Park, D.-H.; Kwak, H.-B.; Kang, J.-H. Roles of myokines in exercise-induced improvement of neuropsychiatric function. Pflugers Arch. 2019, 471, 491–505.
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic Exercise Training Increases Brain Volume in Aging Humans. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2006, 61, 1166–1170.
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009, 19, 1030–1039.
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643.
- Voss, M.W.; Erickson, K.I.; Prakash, R.S.; Chaddock, L.; Kim, J.S.; Alves, H.; Szabo, A.; Phillips, S.M.; Wójcicki, T.R.; Mailey, E.L.; et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav. Immun. 2013, 28, 90–99.
- Vreugdenhil, A.; Cannell, J.; Davies, A.; Razay, G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: A randomized controlled trial. Scand. J. Caring Sci. 2012, 26, 12–19.
- Aarsland, D.; Sardahaee, F.S.; Anderssen, S.; Ballard, C. The Alzheimer’s Society the Alzheimer’s Society Systematic Review group Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment. Health 2010, 14, 386–395.
- Blondell, S.J.; Hammersley-Mather, R.; Veerman, J.L. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014, 14, 510.
- Amboni, M.; Barone, P.; Hausdorff, J.M. Cognitive contributions to gait and falls: Evidence and implications. Mov. Disord. 2013, 28, 1520–1533.
- Kramer, A.F.; Colcombe, S. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study—Revisited. Perspect. Psychol. Sci. 2018, 13, 213–217.
- Mokhtarzade, M.; Motl, R.; Negaresh, R.; Zimmer, P.; Khodadoost, M.; Baker, J.S.; Patel, D.; Majdinasab, N.; Ranjbar, R. Exercise-induced changes in neurotrophic factors and markers of blood-brain barrier permeability are moderated by weight status in multiple sclerosis. Neuropeptides 2018, 70, 93–100.
- Swain, R.; Harris, A.; Wiener, E.; Dutka, M.; Morris, H.; Theien, B.; Konda, S.; Engberg, K.; Lauterbur, P.; Greenough, W. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 2003, 117, 1037–1046.
- Van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270.
- Van Praag, H. Neurogenesis and exercise: Past and future directions. Neuromolecular Med. 2008, 10, 128–140.
- Tari, A.R.; Norevik, C.S.; Scrimgeour, N.R.; Kobro-Flatmoen, A.; Storm-Mathisen, J.; Bergersen, L.H.; Wrann, C.D.; Selbæk, G.; Kivipelto, M.; Moreira, J.B.N.; et al. Are the neuroprotective effects of exercise training systemically mediated? Prog. Cardiovasc. Dis. 2019, 62, 94–101.
- Widmann, M.; Nieß, A.M.; Munz, B. Physical Exercise and Epigenetic Modifications in Skeletal Muscle. Sports Med. 2019, 49, 509–523.
- Iizuka, K.; Machida, T.; Hirafuji, M. Skeletal Muscle Is an Endocrine Organ. J. Pharmacol. Sci. 2014, 125, 125–131.
