Plasmonic biosensing has enabled the design of novel detection strategies capable of targeting individual molecules while evaluating their binding affinity and biological interactions.
- single-molecule analysis
- biosensors
- plasmonics
- nanoparticle
- nanostructure
- nucleic acids
- virus
- living-cells
1. Introduction
The detection of clinical biomarkers with relevant sensitivity is becoming a great concern for biomedical analysis [1,2,3]. The objective quantification of biological targets (i.e., nucleic acids, proteins, cells and microorganisms) enables both the identification of normal or pathological diagnostic outcomes and the monitoring of the biological response to therapeutic drugs or environmental agents. Typically, chemical and biological species are present in clinical samples at trace concentrations as low as attomolar levels [1,2,4]. Measuring such small amounts of biomolecules is particularly relevant not only for the early identification of cancer biomarkers but also for the rapid recognition of pathogenic agents and the prevention of potential outbreaks (Figure 1) [2,5]. For instance, the precise diagnosis of bacterial and virus infections such as tuberculosis, hepatitis, Acquired Immune Deficiency Syndrome (AIDS) and more recently coronavirus disease 2019 (COVID-19) may require the transduction of independent events occurring at single-molecule or cellular levels [2,4]. Single-molecule analysis is also needed when only a minimum volume of sample is available or multiplexed analysis is demanded to target various analytes simultaneously in a short time [3,6].
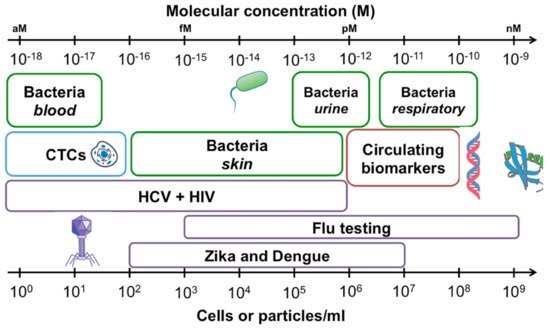
Figure 1. Concentrations of common analytes in biological samples expressed as cells or particles per milliliter of sample (bottom axis), and molarity (top axis). Bacterial concentrations are outlined in green, viral targets are outlined in purple, circulating tumor cells (CTCs) are shown in blue and circulating biomarkers including proteins and nucleic acids in red. Reprinted with permission from Kelley et al. [2] Copyright © 2017 American Chemical Society.
Meeting these challenges involves the monitoring of single-molecule interactions between reacting species via sensing devices that can distinguish binding kinetics in heterogeneous samples with sufficient specificity [1,4]. The observation of a signal from an individual molecule within the ensemble also relies on the transport of the analytes to the sensing region. Therefore, strategies to improve single molecule detection should take into consideration both the sensing mechanism and the diffusion rate, analyte concentration and sample volume [6].
Current research in single-molecule bioanalytical technologies has been primarily focused on configurations based on total internal reflection fluorescence (TIRF) and fluorescent-related methods [1,7,8]. Other optical detection techniques such as surface-enhanced Raman scattering (SERS), total internal reflection scattering (TIRS), dark-field microscopy (DFM), or near-field scanning optical microscopy (NSOM) have also demonstrated their value for the determination of single-molecule interactions [9,10].
From this perspective, plasmonic-based techniques take advantage of the unique optical and electronic properties of plasmonic nanostructures to assess the signals and kinetic distributions of single-molecule binding events occurring in real-time [11,12]. Nanoplasmonic sensing principles rely on the collective oscillation of free electrons known as localized surface plasmons at metallic interfaces or within nanostructures under light stimulation [13,14,15]. The possibility of providing sensitive and selective analysis using single-molecule sensing mechanisms depend on the signal amplification resulting from the enhancement of the intensity of the optical field [8]. Therefore, the manipulation of the physicochemical environment and composition of plasmonic nanostructures allow the precise confinement of the electromagnetic field into nanoscale volumes of only a few nanometers, which are named “hot spots”. The generation of extreme electric field gradients facilitates optical trapping of single molecules while providing longer dwelling times [16,17]. Particularly, the interaction between a biological receptor and its target analyte near the optical-field can be detected through the shift of the plasmon polariton resonance wavelength induced by a local refractive index change [13].
The design of high-spatial-resolution surface structures is very valuable for the surface-enhanced sensing of individual events (Figure 2) [18]. In this sense, plasmonic single-molecule approaches mainly rely upon the extinction spectra of metallic nanoparticles (NPs) and the capacity of optical nanoapertures (i.e., arrays of nanoholes, nanopores, nanowells) to confine the electromagnetic field into ultrasmall subwavelength dimensions [1,8,9,19,20,21,22,23]. Both types of plasmonic nanostructures have been successfully applied to the label-free quantification of single-proteins in the diagnosis of acute and chronic diseases in early stages, the recognition of single-nucleotide specificity and the tracking of independent biological processes in living cells inserted in nanopores [24,25,26].
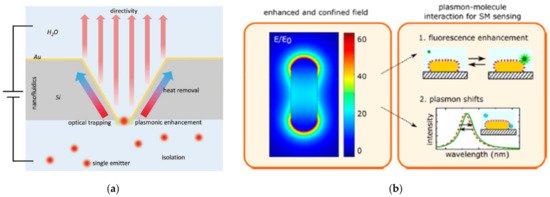
Figure 2. Schematic representation of plasmon-enhanced single-molecule sensing using nanoapertures and nanoparticles: (a) Nanoapertures (rectangle in an etched silicon film) in metal films (gold) for enhanced interaction with single emitters (shown as red glowing circles). The anisotropic etch produces a horn antenna for directive coupling. The aperture provides plasmonic enhancement for optical trapping. The aperture also serves as a nanopore for nanofluidic functionality, like monitoring ionic current from flow through the aperture Adapted with permission from Gordon et al. [27] Copyright © 2020 Wiley; (b) Nanoparticles: The plasmon resonance induces a strongly enhanced and tightly confined local field around the particles. The field shown here is for a gold nanorod that is excited on resonance. The local field mediates plasmon−molecule interactions, enabling enhanced single-molecule detection by monitoring plasmon-induced changes of the molecule (resulting in, e.g., fluorescence enhancement) or by monitoring molecule-induced changes of the plasmon (resulting in frequency-shifts of the plasmon). Adapted with permission from Taylor et al. [4] Copyright © 2017 American Chemical Society.
Although the fundamentals and functionalities of single-molecule biosensing have been extensively addressed in previous reviews [1,8,22], the specific role of plasmonic biosensors in the detection of single-molecule biological targets has still not been satisfactorily resolved. Therefore, the aim of this work is to summarize recent progress in plasmonic single-molecule biosensing schemes for biomedical applications. This review particularly concentrates on the quantification of clinically relevant biomarkers using plasmonic nanostructures such as metallic nanoparticles and optical nanoapertures. Recent research in the design of the new generation of plasmonic platforms for practical sensing of independent molecular interactions from the perspective of point of care testing is also reviewed.
2. Plasmonic Nanoapertures for Single-Molecule Analysis
The exploitation of plasmonic nanoapertures as sensing probes for single-molecule analysis has attracted great interest over the last decade. The formation of nanoapertures in an opaque metal film permits the confinement of light in the subwavelength regime, thereby limiting the interaction volume to the nanometer scale [28,29].
Among nanoscale apertures, solid-state nanopores provide the basis for the development of stable and durable sensing platforms capable of achieving extremely low single-molecule detection limits with high specificity [1,30]. Nanopore sensors consist of an electrically thin insulating membrane traversed by 1–100 nm pores through which ions can cross [22,31]. When charged molecules pass through the pore, the application of a voltage between two electrodes produces the interaction with the membrane and the displacement of ions, causing the change of the ionic current. This displacement can be determined via optical or electrical sensing [1,22]. The optical sensing mainly depends on the change of the refractive index after the arrival of the target molecule to the hotspot of the plasmonic nanopore, wherein the electromagnetic field is strongly localized (Figure 3) [19]. Specifically, the optical transmission through the nanoaperture conducts the plasmon resonance to the far field in which the individual molecule can be detected. Monitoring of the plasmon resonance redshift can either be performed by registering the plasmon resonance peak or by tracking the scattered light intensity at a fixed excitation wavelength. In this way, plasmonic nanoapertures enable the ultrasensitive quantification of molecular interactions by combining the acquisition of bimodal optical and electrical data [32].
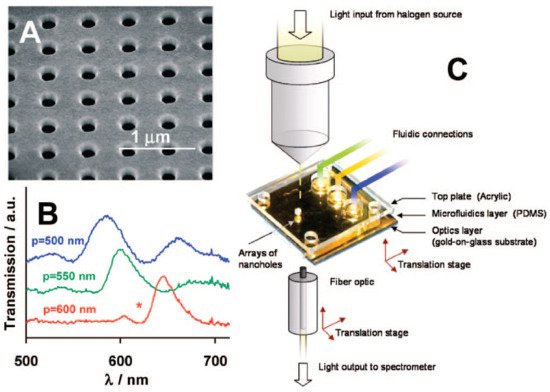
Figure 3. (A) Scanning electron micrograph (SEM) of an array of nanoholes in a gold film; (B) Extraordinary Optical Transmission (EOT) spectra for three arrays with different periodicities; (C) experimental setup to measure the EOT effect. The metal film is deposited on a glass slide, and the gold side of the array is exposed to solvents and aqueous solutions delivered by microfluidics. Adapted with permission from Gordon et al. [19] Copyright © 2008 American Chemical Society.
Since nanopore sensors facilitate the active transport of charged molecules at very low concentrations towards the nanopore aperture from long distances, the combination with plasmonic sensing can contribute to improve the diffusion of single-molecules at a plasmonic hotspot. In addition, the nanoscale diameter of the nanopore is comparable to the cross-section of the molecule, thus reducing the molecule motion to only one dimension. This feature allows both the increment of the dwelling time and the enhancement of the signal-to-background ratio [22]. Therefore, by controlling the nanopore diameter, analytes can be confined to a restricted space and the capture time increases significantly. Accordingly, the reduction in the nanopore size contributes to enhancing the signal-to-noise ratio results enabling highly selective single-molecule detection.
These interesting properties have paved the way for a variety of biological applications aimed at manipulating nanoscale molecules such as single-proteins, virus like-particles or nucleic acids. A detailed description of the fabrication of nanopores has been comprehensively reviewed in previous works and it is beyond the scope of this review [22,33]. Novel developments in plasmonic nanopore sensing schemes for evaluating biological processes including protein-protein interactions, translocation of single proteins, assessment of kinetic reactions, analysis of RNA structure, single nucleotide polymorphisms and DNA sequencing approaches are shown in this section (see Table 1).
Table 1. Key analytical features of plasmonic applications based on nanopores and nanohole arrays sensors classified according to the characteristics of target analyte, instrument configuration (namely sensing scheme or biological receptor) and detection format.
| Target Analyte | Instrument Configuration | Detection Strategy | Reference |
|---|---|---|---|
| Nucleic acids | Nanopore-nanowells with fluorescence enhancement | Double-stranded single DNA molecules adsorption | [34] |
| Graphene quantum dots with a nanopore (density functional theory calculations.) | DNA-graphene interactions and nucleobase (adenine, thymine, cytosine, and guanine) rotation | [35] | |
| Nanopore, bowtie, and bowtie-nanopore structures and SPR materials (chromium, aluminum, rhodium and graphene) | DNA nucleotides shifts in the SPR spectra through Bowtie-nanopore structures | [36] | |
| Two elongated nanodiscs in a gold bowtie nanoantenna | Single-DNA molecules in the nanopore gap monitoring (l intensity of light backscattered from the antenna) | [37] | |
| Inverted-bowtie gold plasmonic nanopore | DNA-protein interactions and DNA translocation (optical signals under different illumination conditions) | [38] | |
| Nanohole arrays | Single-DNA origami triangles were captured on SiO2 bottom surface of gold nanoholes | [39] | |
| Proteins | Plasmonic nanopore tri-color fluorescence simulation and pattern-recognition algorithms | Individual protein translocations, human plasma proteome and cytokine recognition | [40] |
| Plasmonic nanopore acting as nano tweezer | Individual beta-amylase proteins optical trapping | [41] | |
| Infectious agents | Optical nanoantenna with direct physical fluorescence amplification | Single nucleotide variations in Zika artificial DNA origami buffer and human serum | [42] |
| Nanohole array | Single dengue virus-like particles and virucidal drug candidates (spectral shifts of transmission peaks) | [43] | |
| Organic compounds | Silver nanohole arrays and microscopy imaging | Individual rhodamine 6G (R6G) three-dimensional orientation | [44] |
(References would be added automatically after the entry is online)
This entry is adapted from the peer-reviewed paper 10.3390/bios11040123
