Conducting polymers (CPs) are unique due to their ease of synthesis, environmental stability, and simple doping/dedoping chemistry. Electrically conductive silicone polymers are the current state-of-the-art for, e.g., optoelectronic materials.
- silicon-containing polymers
- silane
- silole
1. Introduction
Silicon, as the second most common element in the earth’s crust, forms bonds mainly with oxygen (siloxanes), hydrogen (silanes), and carbon (silanes and siloles). Due to their physicochemical properties, silicone structures have found wide application in materials science [1], optoelectronic technology [2], medicine [3], and environmental protection [4]. Compared to carbon and its bonds, e.g., with hydrogen, the Si–H bond is polarized in such way that silicon has a positive character and hydrogen has a hydride character [5]. As a result, silanes undergo heterolytic reactions more easily than alkanes. Moreover, Si–H and Si–Si bonds are weaker than their carbon analogs, while Si–X bonds (X = N, O, S, F, Cl, Br, and I) are much stronger than C–X bonds. Another difference is the instability of hydrosilicons, given the thermodynamic and kinetic results, compared to hydrocarbons [6][7]. This stability can be increased by introducing organic groups (Si–C bonds instead of Si–H). Furthermore, the Si–Si bond, due to the presence of delocalized δ-electrons, can be compared to the C = C bond and delocalization of π-electrons [8]. The siloxanes have a Si–O bond with a length of 1.64 Å, which is significantly longer than that of the C–C bond (1.53 Å). Moreover, the Si–O–Si bond angle is approximately 143°, which is much larger than the tetrahedral angle (~110°). The significant fact is that the Si–O–Si bond angle is so flexible that it can easily pass through the linear 180° state [9]. Furthermore, the torsional potential of Si–O bonds is significantly lower than that of C–C bonds. Due to these properties, siloxanes are flexible chains and have high thermal stability [10].
Recently, silanes and siloles have been used as promising building blocks for functional organic materials, since silicone-containing π-conjugated compounds are endowed with efficient electron-transporting properties and/or high quantum yields. Due to the direct relationship between the electronic structure of π-conjugated heteroaromatic systems and optoelectric properties, compounds with specific parameters can be designed. By adjusting the highest occupied molecular orbital (HOMO)/lowest unoccupied molecular orbital (LUMO) energy levels, one can control the length of the π-coupling, the frequency of light emission, as well as the injection of the charge. The silicon-containing polymers with multifunctional properties and the ease of their modification by chemical synthesis, using the most common polycondensation reactions based on Suzuki-Miyaury, Stille coupling, Heck, and Sonogashira reactions, have a wide range of potential applications in optoelectronics [11], gas separation membranes [12], porous organic frameworks [13], surface coatings [14], and sensors [15].
2. Arylsilanes—The Most Commonly Used Syntheses
Recently, a lot of attention has been paid to arylsilanes, because silicon can serve as a link between moieties in a molecule and also between organic and inorganic materials [16][17]. Such materials, due to the higher ionic character of the C–Si bond compared to the C–C bond, have greater thermal stability [18]. Moreover, molecules based on silane core have been proven to be building blocks for constructing various 3D porous metal-organic frameworks (MOFs), dynamic covalent frameworks (DCFs), element-organic framework (EOF), and dendrimer-like structures [19][20][21]. The next important group of silanes is the group of tetra-arylsilane (TPS) derivatives, due to their wide energy gap, triplet energy level, and high electrochemical, thermal, and morphological stability [22][23]. More importantly, with its higher solubility used in low-cost processing techniques, such as spin-coating and inkjet printing, desirable for large-area applications, TPS is a promising building material on an industrial scale [24][25][26].
In the synthesis of arylsilanes, palladium-catalyzed coupling reactions (e.g., Suzuki and Stille reactions) and Grignard reactions are most often used [23][27][28][29]. In order to obtain silane intermediates in the above reactions, the most common is the metalation reaction with the addition of chlorosilane additionally substituted with an aryl group or an alkyl chain [30][31][32].
This solution was used by R. Yang et al. [33]. The targeted compounds named TCzSi and DCzMzSi were synthesized by a palladium-catalyzed Suzuki–Miyaura coupling reaction (Scheme 1). The first stage was based on a metalation reaction with the addition of phenyltrichlorosilane. The results indicate that the typical tetrahedral geometry owing to the coordination of silicon was effective in ensuring the separation of the three functional units in a molecule and lead to a high triplet energy level (ET) and relatively wide band gap (Eg).
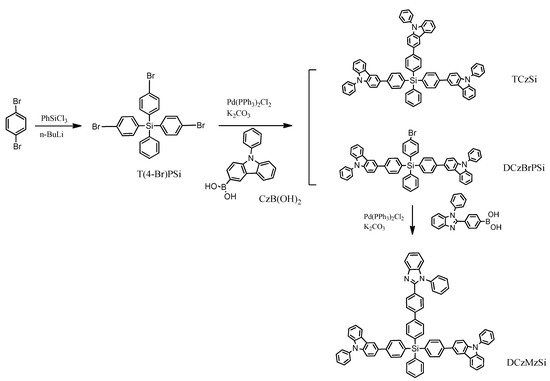
Scheme 1. The synthetic route to TCzSi and DCzMzSi [33].
O. Bezvikonnyi and co-workers obtained 9,9′-bis(triphenylsilyl)-9H,9′H-3,3′-bicarbazole (BiCzSiPh3) in a two-step synthesis [34]. The first step, the synthesis of 3,3′-bicarbazole, was a Friedel–Crafts reaction with FeCl3 as Lewis acid. This method allows the synthesis of 3,3′-bicarbazole without using column chromatography. Compound BiCzSiPh3 was synthesized by standard treatment of the intermediate with n-BuLi, and then the reaction was quenched with chlorotriphenylsilane.
3. Siloles—The Most Commonly Used Syntheses
Siloles possess a low-lying LUMO level, due to the σ*–π* conjugation between the π* orbital of the butadiene moiety and the σ* orbital of the two exocyclic σ-bonds on the silicon atom [35]. Furthermore, the silicon atom stabilizes the highest occupied molecular orbital; thus, siloles exhibit high thermodynamical stability in the air [36]. Moreover, aggregation-induced emission (AIE) has been observed in silole [37]. Phenyl substituted siloles and polysilanes can be considered examples of mixed π-conjugation/cross-hyperconjugation and π-conjugation/σ-conjugation, respectively.
The synthesis of dithiensiloles, as in the case of arylsiloles, is based on C–C coupling reactions, e.g., the Stille and Sonogashira reactions [38][39]. Similarly, silole-containing intermediates are synthesized by metalation in reaction with n-BuLi or reductive cyclization with lithium naphthalenide [38][40].
A. Pöcheim et al. attempt to show that the combination of siloles as the prototypical examples of cross-hyperconjugated molecules and oligosilanes, representative of σ-conjugation, forms systems of extended conjugation, leading to altered optical absorption properties [41]. Researchers decided to use di(phenylalkynyl)silane 37 as a starting material (Scheme 2). Reductive cyclization with lithium naphthalenide was followed by a reaction with excess dichlorodimethylsilane to the 2,5-chlorodimethylsilyl substituted silole 38. If chlorotrimethylsilane was used instead of dichlorodimethylsilane, the 2,5-bis(trimethylsilyl) substituted silole 40 was obtained. The chlorodimethylsilyl substituents of 38 provide the possibility to introduce oligosilanyl groups to the 2,5-positions. A reaction with potassium tris(trimethylsilyl)silanide led to a formation of the neopentasilanyl substituted silole 39. Compound 38 was also reacted with 1,1,4,4,4-pentakis(trime-thylsilyl)tetramethyltetrasilanylpotassium to yield compound 41 with attached oligosilanyl fragments containing linear hexasilane units.
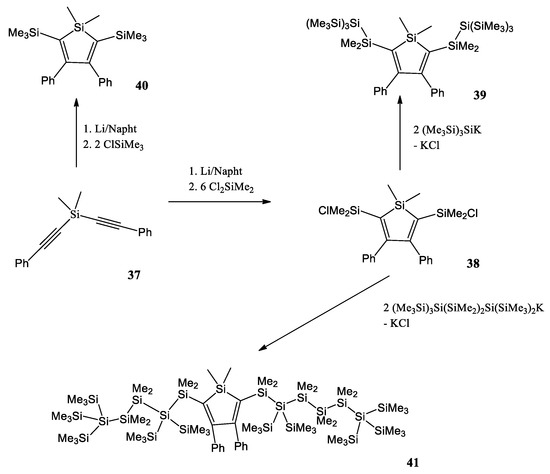
Scheme 2. Synthesis of a variety of 2,5-disilyl substituted siloles [41].
This entry is adapted from the peer-reviewed paper 10.3390/molecules26072012
References
- Arukalam, I.O.; Oguzie, E.E.; Li, Y. Nanostructured superhydrophobic polysiloxane coating for high barrier and anticorrosion applications in marine environment. J. Colloid Interface Sci. 2018, 512, 674–685.
- Tang, X.; Yao, L.; Liu, H.; Shen, F.; Zhang, S.; Zhang, H.; Lu, P.; Ma, Y. An Efficient AIE-Active Blue-Emitting Molecule by Incorporating Multifunctional Groups into Tetraphenylsilane. Chem. Eur. J. 2014, 20, 7589–7592.
- Shang, Y.; Zheng, N.; Wang, Z. Tetraphenylsilane-Cored Star-Shaped Polymer Micelles with pH/Redox Dual Response and Active Targeting Function for Drug-Controlled Release. Biomacromolecules 2019, 20, 4602–4610.
- Ma, J.; Xia, W.; Zhang, R.; Ding, L.; Kong, Y.; Zhang, H.; Fu, K. Flocculation of emulsified oily wastewater by using functional grafting modified chitosan: The effect of cationic and hydrophobic structure. J. Hazard. Mater. 2021, 403, 123690.
- Koda, S. Kinetic aspects of oxidation and combustion of silane and related compounds. Prog. Energy Combust. Sci. 1992, 18, 513–528.
- Walsh, R. Bond dissociation energy values in silicon-containing compounds and some of their implications. Acc. Chem. Res. 1981, 14, 246–252.
- Walsh, R. Thermochemistry of silicon-containing compounds. Part 1—Silicon–halogen compounds, an evaluation. J. Chem. Soc. Faraday Trans. 1 1983, 79, 2233–2248.
- Semenov, V.V. Alkanes and silanes: Similarities and differences. Her. Russ. Acad. Sci. 2016, 86, 466–472.
- Mark, J.E.; Allcock, H.R.; West, R. Inorganic Polymers, 2nd ed.; Oxford University Press: New York, NY, USA, 2004.
- Mark, J.E. Some Interesting Things about Polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953.
- Lee, S.H.; Jang, B.B.; Kafafi, Z.H. Highly Fluorescent Solid-State Asymmetric Spirosilabifluorene Derivatives. J. Am. Chem. Soc. 2005, 127, 9071–9078.
- Ahmad, N.A.; Leo, C.P.; Ahmad, A.L. Amine wetting evaluation on hydrophobic silane modified polyvinylidene fluoride/silicoaluminophosphate zeolite membrane for membrane gas absorption. J. Nat. Gas. Sci. Eng. 2018, 58, 115–125.
- Zaręba, J.K. Tetraphenylmethane and tetraphenylsilane as building units of coordination polymers and supramolecular networks—A focus on tetraphosphonates. Inorg. Chem. Commun. 2017, 86, 172–186.
- Guan, W.W.; Shi, X.Y.; Xu, T.T.; Wan, K.; Zhang, B.W.; Liu, W.; Su, H.L.; Zou, Z.Q.; Du, Y.W. Synthesis of well-insulated Fe–Si–Al soft magnetic composites via a silane-assisted organic/inorganic composite coating route. J. Phys. Chem. Solids 2021, 150, 109841.
- Bomina, S.; Honglae, S. 1,3,5-Trinitrotoluene Sensor Based on Silole Nanoaggregates. J. Nanosci. Nanotechnol. 2019, 19, 991–995.
- Sasi Kumar, R.; Padmanathan, N.; Alagar, M. Design of hydrophobic polydimethylsiloxane and polybenzoxazine hybrids for interlayer low k dielectrics. New J. Chem. 2015, 39, 3995–4008.
- Liu, H.; Fu, Z.; Song, F.; Qingquan, L.; Chen, L. The controllable construction and properties characterization of organic–inorganic hybrid materials based on benzoxazine-bridged polysilsesquioxanes. RSC Adv. 2017, 7, 3136–3144.
- Hsieh, C.Y.; Su, W.C.; Wu, C.S.; Lin, L.K.; Hsu, K.Y.; Liu, Y.L. Benzoxazine-containing branched polysiloxanes: Highly efficient reactive-type flame retardants and property enhancement agents for polymers. Polymer 2013, 54, 2945–2951.
- Feng, D.; Wang, K.; Wei, Z.; Chen, Y.-P.; Simon, C.M.; Arvapally, R.K.; Martin, R.L.; Bosch, M.; Liu, T.-F.; Fordham, S.; et al. Kinetically tuned dimensional augmentation as a versatile synthetic route towards robust metal-organic frameworks. Nat. Commun. 2014, 5, 5723.
- Duncan, N.C.; Hay, B.P.; Hagaman, E.W.; Custelcean, R. Thermodynamic, Kinetic, and Structural Factors in the Synthesis of Imine-Linked Dynamic Covalent Frameworks. Tetrahedron 2012, 68, 53–64.
- Rose, M.; Böhlmann, W.; Sabo, M.; Kaskel, S. Element–organic frameworks with high permanent porosity. Chem. Commun. 2008, 2462–2464.
- Sun, D.; Zhou, X.; Li, H.; Sun, X.; Zheng, Y.; Ren, Z.; Ma, D.; Bryce, M.R.; Yan, S. A versatile hybrid polyphenylsilane host for highly efficient solution-processed blue and deep blue electrophosphorescence. J. Mater. Chem. C 2014, 2, 8277–8284.
- Li, X.; Bai, Q.; Li, J.; Lu, F.; Sun, X.; Lu, P. Synthesis and properties of wide bandgap polymers based on tetraphenylsilane and their applications as hosts in electrophosphorescent devices. New J. Chem. 2018, 42, 3344–3349.
- Moncada, J.; Terraza, C.A.; Tagle, L.H.; Coll, D.; Ortiz, P.; Pérez, G.; de la Campa, J.G.; Álvarez, C.; Tundidor-Camba, A. Synthesis, characterization and studies of properties of six polyimides derived from two new aromatic diamines containing a central silicon atom. Eur. Polym. J. 2017, 91, 354–367.
- Tundidor-Camba, A.; González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Tagle, L.H.; Hauyón, R.A.; Sobarzo, P.A.; González, A.; Ortiz, P.A.; Maya, E.M.; Terraza, C.A. Silylated oligomeric poly(ether-azomethine)s from monomers containing biphenyl moieties: Synthesis and characterization. RSC Adv. 2018, 8, 1296–1312.
- Cai, M.; Xiao, T.; Hellerich, E.; Chen, Y.; Shinar, R.; Shinar, J. High-Efficiency Solution-Processed Small Molecule Electrophosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2011, 23, 3590–3596.
- Oner, S.; Oner, I.; Akda, H.; Varlikli, G.C. Tetraphenylsilane group containing carbazoles as high triplet energy host materials for solution-processable PhOLEDs. Turk. J. Chem. 2015, 39, 917–929.
- Arsenyan, P.; Pudova, O.; Popelis, J.; Lukevics, E. Novel radial oligothienyl silanes. Tetrahedron Lett. 2004, 45, 3109–3111.
- Wang, Y.; Tan, D. Synthesis, thermal degradation and dielectric properties of poly[octyl(triphenylethynyl)]silane resin. Chem. Res. Chin. Univ. 2019, 35, 1076–1081.
- Kai, Z.; Li, H.; Shi, H.; Wu, J.; Xu, J.; Li, Y.; Zhao, C. Synthesis and Thermal Properties of Silicon-Containing Benzoxazine. High Perform. Polym. 2020, 32, 59–64.
- Zając, D.; Sołoducho, J.; Jarosz, T.; Łapkowski, M.; Roszak, S. Conjugated silane-based arylenes as luminescent materials. Electrochim. Acta 2015, 173, 105–116.
- Zając, D.; Sołoducho, J.; Jarosz, T.; Łapkowski, M.; Roszak, S. Push-pull structures of symmetric silane derivatives as a novel hosting materials. Indian J. Appl. Res. 2017, 7, 58–66.
- Yang, R.; Li, D.; Bai, Y.; Zhang, L.; Liu, Z.; Hao, J.; Wei, Q.; Liao, L.; Peng, R.; Ge, Z. Novel tetraarylsilane-based hosts for blue phosphorescent organic light-emitting diodes. Org. Electron. 2018, 55, 117–125.
- Bezvikonnyi, O.; Grybauskaite-Kaminskiene, G.; Volyniuk, D.; Simokaitiene, J.; Danyliv, Y.; Durgaryan, R.; Bucinskas, A.; Jatautienė, E.; Hladka, I.; Scholz, J.; et al. 3,3′-Bicarbazole-based compounds as bipolar hosts for green and red phosphorescent organic light-emitting devices. Mater. Sci. Eng. B 2020, 261, 114662.
- Kim, D.H.; Ohshita, J.; Lee, K.H.; Kunugi, Y.; Kunai, A. Synthesis of π-Conjugated Oligomers Containing Dithienole Units. Organometallics 2006, 25, 1511–1516.
- Grisorio, R.; Suranna, G.P.; Mastrorilli, P.; Allegretta, G.; Loiudice, A.; Rizzo, A.; Gigli, G.; Manoli, K.; Magliulo, M.; Torsi, L. All-donor poly(arylene-ethynylene)s containing anthracene and silole-based units: Synthesis, electronic, and photovoltaic properties. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4860–4872.
- Chen, J.; Xie, Z.; Lam, J.W.Y.; Law, C.C.W.; Tang, B.Z. Silole-Containing Polyacetylenes. Synthesis, Thermal Stability, Light Emission, Nanodimensional Aggregation, and Restricted Intramolecular Rotation. Macromolecules 2003, 36, 1108–1117.
- Zając, D.; Honisz, D.; Łapkowski, M.; Sołoducho, J. Synthesis and Properties of New Dithienosilole Derivatives as Luminescent Materials. Molecules 2019, 24, 2259.
- Feng, Y.; Dai, C.; Lei, J.; Ju, H.; Cheng, Y. Silole-Containing Polymer Nanodot: An Aqueous Low-Potential Electrochemiluminescence Emitter for Biosensing. Anal. Chem. 2016, 88, 845–850.
- Horst, S.; Evans, N.R.; Bronstein, H.A.; Williams, C.K. Synthesis of fluoro-substituted silole-containing conjugated materials. J. Polym. Sci. A Polym. Chem. 2009, 47, 5116–5125.
- Pöcheim, A.; Özpınar, G.A.; Müller, T.; Baumgartner, J.; Marschner, C. The Combination of Cross-Hyperconjugation and σ-Conjugation in 2,5-Oligosilanyl Substituted Siloles. Chem. Eur. J. 2020, 26, 17252–17260.
