Neuroactive steroids are a family of all steroid-based compounds, of both natural and synthetic origin, which can affect the nervous system functions. Their biosynthesis occurs directly in the nervous system (so-called neurosteroids) or in peripheral endocrine tissues (hormonal steroids). Steroid hormone levels may fluctuate due to physiological changes during life and various pathological conditions affecting individuals.
- immunoassay
- mass spectrometry
- metabolomics
- neuroactive steroids
- steroid
1. Introduction
Neuroactive steroids (NASs) can be characterized as substances of steroid origin that can have effects on the nervous system [1]. They include hormonal steroids which originated in the peripheral glands, steroids locally synthesized by neurons and glial cells, and synthetic steroids that modify the activity of the nervous system [2].
Their core structure, as for other steroids, is represented by sterane or cyclopentanoperhydrofenanthrene [3] (Figure 1). Members of this group, such as progesterone, estrogens, testosterone, dehydroepiandrosterone (DHEA), or cortisol, are involved in shaping the structure and function of the central nervous system throughout the life cycle [4]. The nervous system is affected by both endogenously synthesized NASs and steroids of exogenous origin [5]. The first location for NAS steroidogenesis is the peripheral endocrine gland. However, the biosynthesis of these substances can also occur directly in the central and peripheral nervous systems, based on which this specific subgroup is referred to as neurosteroids [6]. Steroids of exogenous origin include substances prepared synthetically. The regulation of many processes in the body is based on the ability of NASs to interact with different types of receptors [7]. In particular, these are γ-aminobutyric acid (GABA) receptors, N-methyl-D-aspartate (NMDA) receptors, voltage-gated calcium channels, voltage-dependent anion channels, serotonin receptors, microtubule-associated protein 2, and others.
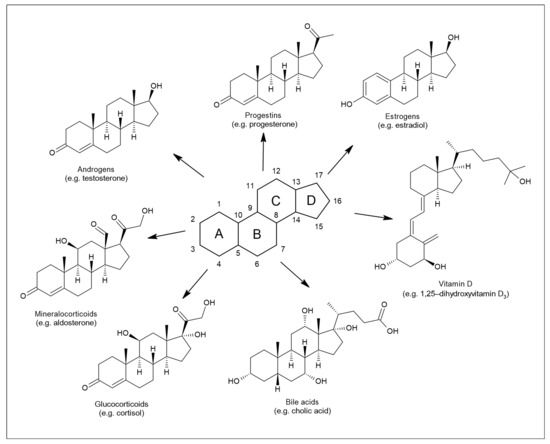
Figure 1. Structure of sterane and seven principal classes of steroid substances.
Many metabolites of sex hormones and some stress hormones act in the central nervous system through the so-called nongenomic mechanism, the effect of which is manifested within a period ranging from a few milliseconds to seconds [8] (Figure 2). These rapid nongenomic effects are made possible due to the interaction of NASs with ion channels and membrane receptors [9]. In contrast, when steroids interact with nuclear or cytoplasmic receptors, the resulting effect occurs after a longer period of time [10]. This is because these intracellular receptors function as transcription factors and are involved in gene expression [11]. NASs can thus affect the function of neurons, as well as other cells of the brain, such as astrocytes, microglia, oligodendrocytes, and endothelial cells [1]. NASs are likely involved in the regulation of neurogenesis, neuron survival, neuritogenesis, glial cell differentiation, myelin formation, and synaptic plasticity. Their neuroprotective effects and their ability to suppress nerve tissue inflammation are also described. For a wide range of neurological and psychiatric diseases, such as schizophrenia, epilepsy, depression, or multiple sclerosis, these substances play an important role in their pathology and therapy [11]. Hormonal differences between men and women are noticeable under both physiological and pathological conditions [1]. It has already been shown that neurodegenerative diseases, such as multiple sclerosis, can affect the levels of circulating NASs [12]. Monitoring these changes in blood plasma or cerebrospinal fluid (CSF) can serve as a warning signal (biomarkers) to draw attention to pathological processes taking place in the nervous system.
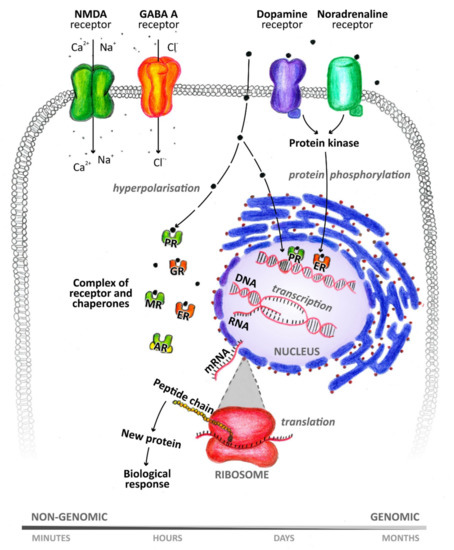
Figure 2. Genomic and nongenomic mechanism of neuroactive steroid action. PR: progesterone receptor, GR: glucocorticoid receptor, MR: mineralocorticoid receptor, ER: estrogen receptor, AR: androgen receptor.
Knowledge of NAS formation and their correct detection can be used to prevent and treat some neurodegenerative and psychiatric diseases. The aim of this article is to provide an overview of the most important methods for NAS analysis, with an emphasis on immunoanalytical methods and gas (GC), or liquid chromatography (LC) combined with mass spectrometry (MS).
2. Determination of Neuroactive Steroids
Efforts to quantify steroid hormones using colorimetry-based methods have existed since the 1930s [13][14][15]. One of the first methods used to define and quantify hormonal activity was also a bioassay [16]. The so-called whole animal in vivo bioassays used before the introduction of radioimmunoassays (RIAs) were arduous and insensitive [17]. Despite large advances in molecular–genetic methods (e.g., PCR, DNA sequencing), determining the metabolic profile of NASs is still important [18]. The information obtained can be used to diagnose, but also to monitor, the course of a disease. Immunoassay- (IA) and MS-based methods are currently used to monitor steroid hormone levels [19]. They provide higher sensitivity, specificity, and reliability. RIAs and direct immunoassays (DIA) dominate among IA approaches.
2.1. Types of Biological Matrices
To determine steroid hormones in clinical practice, blood serum and plasma samples are mainly used [20][21][22][23][24][25]. Moreover, saliva can be an alternative biological material. The advantages of this type of sample are that it is noninvasive, less stressful, and easier to collect compared to blood. Saliva sampling can also be performed in a home environment. In addition, there is a correlation between levels of unconjugated steroid hormones in saliva and levels of their unbound fraction in blood serum. The final levels may differ, which is a consequence of steroid metabolism in the salivary glands. The ratio of cortisol concentrations in saliva and blood serum is around 1:20 and, for testosterone and estradiol, it approximates to 1:90. Furthermore, steroids can be analyzed in other biological matrices, such as urine, CSF, hair, or nails [26][27][28][29][30][31][32]. Urinary steroid profiling has been an integral part of the diagnosis of steroid metabolism disorders for several decades (since the 1960s) [33]. Great interest is focused on the analysis of steroids in human CSF [12][32][34][35][36]. CSF is the only matrix that can be obtained from living donors and it allows for the monitoring of brain metabolism. As mentioned above, the nervous system is a source of neurosteroids. Central neurosteroids are studied as potential biomarkers of various cognitive disorders (e.g., dementia, depression). Extensive research in and use of this matrix are complicated by its limited availability, due to the complexity of collection (usually lumbar puncture), the limitations of its indication in healthy individuals (very small participant groups), as well as its limited sample amount for analysis. Due to the trace levels of neurosteroids in CSF, great demands are also placed on analytical techniques. The interested reader is referred to the cited reference for detailed description of CSF analysis [34].
2.2. Factors Affecting Steroid Hormone Levels
The results of NAS analysis are affected by several important factors, such as age, sex, and, of course, sampling time [19]. A natural decrease in the levels of certain steroids in relation to male age has been found. A similar trend can be observed for women; however, hormonal changes also occur during the menstrual cycle. A decrease in testosterone levels with increasing age in men and women has also been determined in saliva [24]. Levels of some steroid hormones in humans fluctuate during the day (e.g., cortisone, cortisol, corticosterone, 11-deoxycortisol, androstenedione, 17α-hydroxyprogesterone, DHEA, testosterone) [37][38]. Therefore, it is important to take samples under standardized conditions. Boyce and co-workers (2004) published different reference ranges for serum testosterone levels in men depending on the time of day [39]. The range for morning testosterone concentrations is 10.07–38.76 nmol.L−1 for men under 40 years, and 7.41–24.13 nmol.L−1 for men over 40 years of age. The evening ranges are then 6.69–31.51 nmol.L−1 and 6.46–21.93 nmol.L−1, respectively. Salivary testosterone levels for men and women even differ depending on the season [24]. Keevil and co-workers (2017) found the lowest levels of salivary testosterone for men, and the highest for women, in the summer (June–August). In addition to age, sex, and time of day, total testosterone levels are also affected by various pathological conditions [40].
This entry is adapted from the peer-reviewed paper 10.3390/biom11040553
References
- Melcangi, R.C.; Giatti, S.; Garcia-Segura, L.M. Levels and actions of neuroactive steroids in the nervous system under physiological and pathological conditions: Sex-specific features. Neurosci. Biobehav. Rev. 2016, 67, 25–40.
- Giatti, S.; Garcia-Segura, L.M.; Barreto, G.E.; Melcangi, R.C. Neuroactive steroids, neurosteroidogenesis and sex. Prog. Neurobiol. 2019, 176, 1–17.
- Greaves, R.F.; Jevalikar, G.; Hewitt, J.K.; Zacharin, M.R. A guide to understanding the steroid pathway: New insights and diagnostic implications. Clin. Biochem. 2014, 47, 5–15.
- Belvederi Murri, M.; Fanelli, F.; Pagotto, U.; Bonora, E.; Triolo, F.; Chiri, L.; Allegri, F.; Mezzullo, M.; Menchetti, M.; Mondelli, V.; et al. Neuroactive steroids in first-episode psychosis: A role for progesterone? Schizophr. Res. Treat. 2016, 2016, 1–6.
- Melcangi, R.C.; Garcia-Segura, L.M.; Mensah-Nyagan, A.G. Neuroactive steroids: State of the art and new perspectives. Cell. Mol. Life Sci. 2008, 65, 777–797.
- Baulieu, E.-E. Steroid hormones in the brain: Several mechanisms? In Steroid Hormone Regulation of the Brain; Pergamon, Press: Oxford, UK, 1981; pp. 3–14.
- Tuem, K.B.; Atey, T.M. Neuroactive steroids: Receptor interactions and responses. Front. Neurol. 2017, 8, 1–10.
- Wang, M. Neurosteroids and GABA-A Receptor function. Front. Endocrinol. 2011, 2, 1–23.
- Reddy, D.S. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 2010, 186, 113–137.
- Holst, J.P.; Soldin, O.P.; Guo, T.; Soldin, S.J. Steroid hormones: Relevance and measurement in the clinical laboratory. Clin. Lab. Med. 2004, 24, 105–118.
- Zheng, P. Neuroactive steroid regulation of neurotransmitter release in the CNS: Action, mechanism and possible significance. Prog. Neurobiol. 2009, 89, 134–152.
- Caruso, D.; Melis, M.; Fenu, G.; Giatti, S.; Romano, S.; Grimoldi, M.; Crippa, D.; Marrosu, M.G.; Cavaletti, G.; Melcangi, R.C. Neuroactive steroid levels in plasma and cerebrospinal fluid of male multiple sclerosis patients. J. Neurochem. 2014, 130, 591–597.
- Cohen, S.L.; Marrian, G.F. The application of the Kober test to the quantitative estimation of oestrone and oestriol in human pregnancy urine. Biochem. J. 1934, 28, 1603–1614.
- Cohen, H.; Bates, R.W. A simple quantitative colorimetric method for estrogenic steroids. J. Clin. Endocrinol. Metab. 1947, 7, 701–707.
- Pincus, G.; Wheeler, G.; Young, G.; Zahl, P.A. The colorimetric determination of urinary estrin. J. Biol. Chem. 1936, 116, 253–266.
- Auchus, R.J. Steroid assays and endocrinology: Best practices for basic scientists. Endocrinology 2014, 155, 2049–2051.
- Handelsman, D.J. Mass spectrometry, immunoassay and valid steroid measurements in reproductive medicine and science. Hum. Reprod. 2017, 32, 1147–1150.
- Wudy, S.A.; Schuler, G.; Sánchez-Guijo, A.; Hartmann, M.F. The art of measuring steroids: Principles and practice of current hormonal steroid analysis. J. Steroid Biochem. Mol. Biol. 2018, 179, 88–103.
- Conklin, S.E.; Knezevic, C.E. Advancements in the gold standard: Measuring steroid sex hormones by mass spectrometry. Clin. Biochem. 2020, 82, 21–32.
- Gröschl, M. Current status of salivary hormone analysis. Clin. Chem. 2008, 54, 1759–1769.
- Li, X.S.; Li, S.; Kellermann, G. Simultaneous determination of three estrogens in human saliva without derivatization or liquid-liquid extraction for routine testing via miniaturized solid phase extraction with LC-MS/MS detection. Talanta 2018, 178, 464–472.
- Cardoso, E.; Persi, G.; González, N.; Tumilasci, O.; Arregger, A.; Burgos, M.; Rodríguez, V.; Molina, A.; Contreras, L.N. Assessment of adrenal function by measurement of salivary steroids in response to corticotrophin in patients infected with human immunodeficiency virus. Steroids 2007, 72, 328–334.
- Wood, P. Salivary steroid assays-research or routine? Ann. Clin. Biochem. 2009, 46, 183–196.
- Keevil, B.G.; Clifton, S.; Tanton, C.; Macdowall, W.; Copas, A.J.; Lee, D.; Field, N.; Mitchell, K.R.; Sonnenberg, P.; Bancroft, J.; et al. Distribution of salivary testosterone in men and women in a british general population-based sample: The third national survey of sexual attitudes and lifestyles (Natsal-3). J. Endocr. Soc. 2017, 1, 14–25.
- Prokai-Tatrai, K.; Bonds, D.; Prokai, L. Simultaneous measurement of 17β-estradiol, 17α-estradiol and estrone by GC–isotope dilution MS–MS. Chromatographia 2010, 71, 311–315.
- Noppe, G.; de Rijke, Y.B.; Dorst, K.; van den Akker, E.L.T.; van Rossum, E.F.C. LC-MS/MS-based method for long-term steroid profiling in human scalp hair. Clin. Endocrinol. 2015, 83, 162–166.
- Shafigullina, Z.R.; Velikanova, L.I.; Vorokhobina, N.V.; Shustov, S.B.; Lisitsin, A.A.; Malevanaia, E.V.; Buinova, M.O.; Bessonova, E.A.; Kirsanov, D.O. Urinary steroid profiling by gas chromatography mass spectrometry: Early features of malignancy in patients with adrenal incidentalomas. Steroids 2018, 135, 31–35.
- Voegel, C.D.; La Marca-Ghaemmaghami, P.; Ehlert, U.; Baumgartner, M.R.; Kraemer, T.; Binz, T.M. Steroid profiling in nails using liquid chromatography-tandem mass spectrometry. Steroids 2018, 140, 144–150.
- Naldi, A.C.; Fayad, P.B.; Prévost, M.; Sauvé, S. Analysis of steroid hormones and their conjugated forms in water and urine by on-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry. Chem. Cent. J. 2016, 10, 30.
- Wozniak, B.; Matraszek-Zuchowska, I.; Zmudzki, J. LC-MS/MS fast analysis of androgenic steroids in urine. Anal. Bioanal. Chem. 2012, 403, 2965–2972.
- Borts, D.J.; Bowers, L.D. Direct measurement of urinary testosterone and epitestosterone conjugates using high-performance liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2000, 35, 50–61.
- Sosvorova, L.; Vitku, J.; Chlupacova, T.; Mohapl, M.; Hampl, R. Determination of seven selected neuro- and immunomodulatory steroids in human cerebrospinal fluid and plasma using LC-MS/MS. Steroids 2015, 98, 1–8.
- Krone, N.; Hughes, B.A.; Lavery, G.G.; Stewart, P.M.; Arlt, W.; Shackleton, C.H.L. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J. Steroid Biochem. Mol. Biol. 2010, 121, 496–504.
- Teubel, J.; Parr, M.K. Determination of neurosteroids in human cerebrospinal fluid in the 21st century: A review. J. Steroid Biochem. Mol. Biol. 2020, 204, 105753.
- Martin, J.; Plank, E.; Jungwirth, B.; Hapfelmeier, A.; Podtschaske, A.; Kagerbauer, S.M. Weak correlations between serum and cerebrospinal fluid levels of estradiol, progesterone and testosterone in males. BMC Neurosci. 2019, 20, 1–6.
- Nguyen, H.P.; Li, L.; Gatson, J.W.; Maass, D.; Wigginton, J.G.; Simpkins, J.W.; Schug, K.A. Simultaneous quantification of four native estrogen hormones at trace levels in human cerebrospinal fluid using liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 54, 830–837.
- Parikh, T.P.; Stolze, B.; Ozarda, Y.; Jonklaas, J.; Welsh, K.; Masika, L.; Hill, M.; DeCherney, A.; Soldin, S.J. Diurnal variation of steroid hormones and their reference intervals using mass spectrometric analysis. Endocr. Connect. 2018, 7, 1354–1361.
- Stolze, B.R.; Gounden, V.; Gu, J.; Abel, B.S.; Merke, D.P.; Skarulis, M.C.; Soldin, S.J. Use of Micro-HPLC-MS/MS Method to Assess Diurnal Effects on Steroid Hormones. Clin. Chem. 2015, 61, 556–558.
- Boyce, M.J.; Baisley, K.J.; Clark, E.V.; Warrington, S.J. Are published normal ranges of serum testosterone too high? Results of a cross-sectional survey of serum testosterone and luteinizing hormone in healthy men. BJU Int. 2004, 94, 881–885.
- Rosner, W.; Auchus, R.J.; Azziz, R.; Sluss, P.M.; Raff, H. Utility, Limitations, and pitfalls in measuring testosterone: An endocrine society position statement. J. Clin. Endocrinol. Metab. 2007, 92, 405–413.
