A kind of biosensor using aptamers as BRE is known as aptasensor. Aptamers are synthetic single-stranded oligonucleotide sequences (RNA or DNA) with high specificity and affinity to bind a variety of target classes including proteins, peptides, drugs, small molecules, whole cells, inorganic and organic molecules, etc.
- aptamer-based sensors
- AuNPs-based aptasensors
- aptamers
1. Introduction
Aptamers are especially screened via an in vitro selection process named SELEX (systematic evolution of ligands by exponential enrichment) [1], in situ SELEX [2], in-silico/computational approaches [3] and some bioinformatic methods to combine both the in silico and in vitro approaches, etc., to identify the best fit for each target analyte [4]. They have several other striking features such as high stability, greater affinity, low molecular weight, easy and reproducible preparation [5]. They undergo a folding process to make a specific three-dimensional structural rearrangement in order to bind with their particular target (Figure 1B). Three-dimensional structure depends on aptamer sequence, primary structure, pH, temperature and buffer composition of the sensor [6]. Target-aptamer interactions mainly depend on the nature of its binding analyte, three-dimensional structure of the aptamer and distribution of their charges [7]. Interaction types between aptamer target complex involve electrostatic interactions, van der Waals forces, shape complementarity, stacking of flat moieties and hydrogen bonding so that their dissociation constants (Kd) values range from pico- to nanomolar levels [8].
Aptamers can also be referred to as “chemical antibodies” since they mimic antibodies in their applications and characters. Although aptamers are advantageous over antibodies in many aspects especially because of their high affinity, strong specificity, low immunogenicity, targeting a broad range of analytes, cost effectiveness and short preparation cycle [9] they are not as commonly used as antibodies, however developing rapidly [10]. Performance of different aptasensors can be compared keeping in view some important characteristics or parameters of their constituent aptamers such as affinity, specificity, selectivity, limit of detection (LOD), and stability, etc.
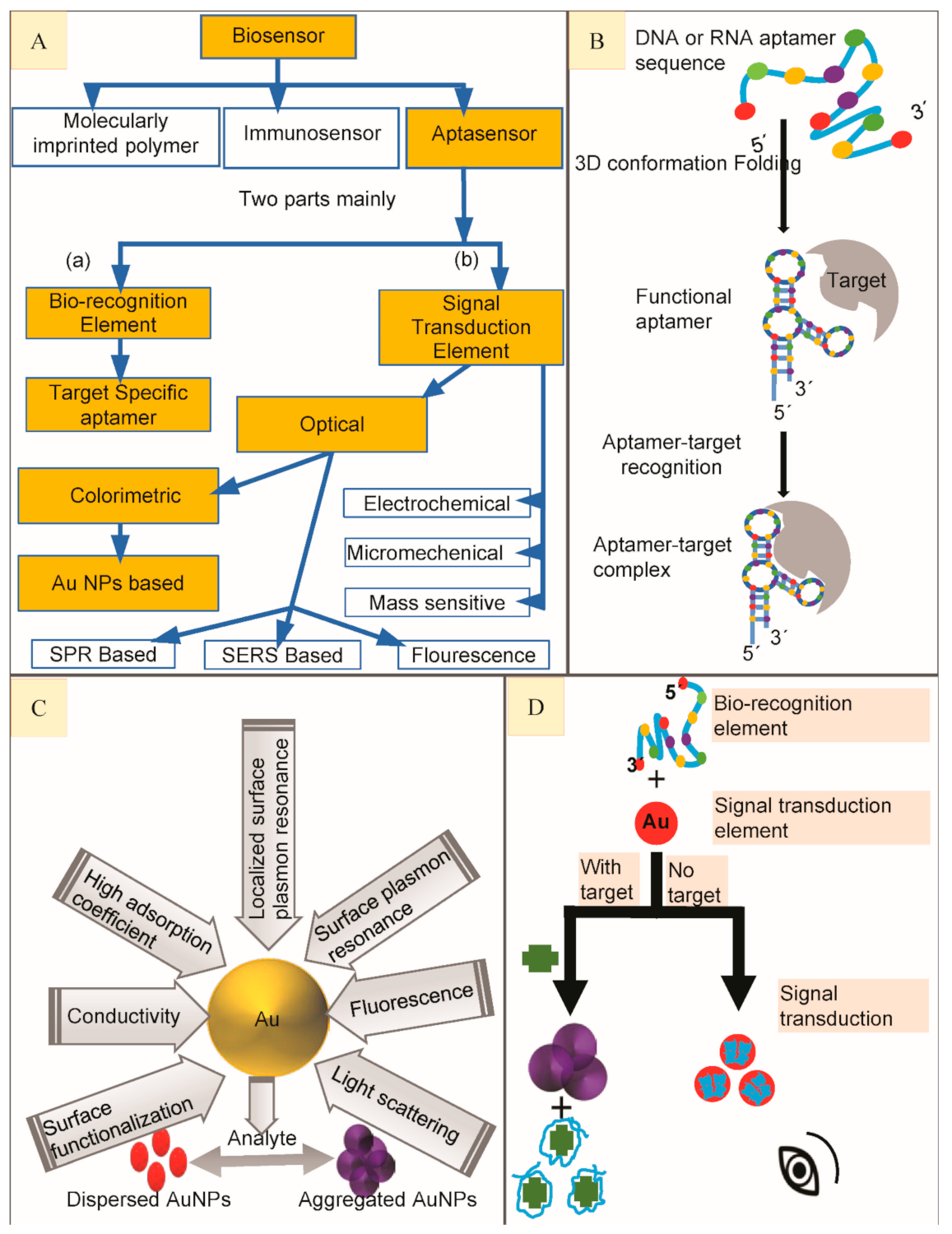
Figure 1. (A) Scheme to show main types of biosensors to highlight aptasensor, classification of aptasensors based on its major components to highlight Gold Nanoparticles (AuNPs) based colorimetric aptasensors. (B) Generalized depiction of an aptamer library folding to form secondary structure in order to bind with high affinity target molecule to finally make aptamer-target complex. (C). Properties of Gold nanoparticles and calorimetry. (D) A generalized illustration on working principle of colorimetric aptasensor when bio-recognition element (BRE) combines with signal transduction element (STE) to make a colorimetric aptasensor to detect small molecular targets.
2. Gold Nanoparticles (AuNPs) Based Colorimetric Aptasensors
Nanomaterials are widely used for their excellent sensing application in biosensing with favorably lowered detection limits and higher sensitives. Almost all nanomaterials take the advantage of high specific surface allowing the immobilization of an enhanced amount of bio-recognition element (BRE) to substitute the classic transduction methods. Covalent linkage of nanomaterials to biomolecules is attributed towards the lowering of unspecific physisorption, stability of the system and the reproducibility of the surface functionalization. A variety of nanomaterials, each with their own unique properties are known to enhance biosensor performance [11].
The combination of aptamers with AuNPs has been widely used to develop colorimetric aptasensors for optical recognition of antibiotics [12]. AuNPs have attracted greater attention owing to their light-scattering properties, strong optical absorption, unique chemical, biological, electronic properties, low or no toxicity and fluorescence quenching, etc. [13][14] (Figure 1C). Their unique optical properties are the result of mutual electronic oscillations at their surface which can be controlled by regulating their sharpness, composition and size (diameter) especially. Their diameter can be easily controlled by various experimental parameters to shape-up the desired optical features [15]. Fabricating colorimetric aptasensors by the use of AuNPs is gradually getting popularity for their simple operation and easy preparation [16]. The STE is an exceedingly essential part of an aptasensor while designing colorimetric assays with AuNPs because of their special optical properties, e.g., high extinction coefficient and surface plasmon resonance (SPR) [17]. The aptamers are adsorbed on AuNPs surface (in the absence of their high-affinity target) to stabilize them, thus preventing their salt-induced aggregation because of SPR connection among adjoining particles [18]. Contrarily, on target addition to the aptasensor, aptamer binds to their high affinity target, causing aptamer desorption from AuNPs surface, thus aggregating the AuNPs leading to a color change from bright red to blue or purple (Figure 1D) most commonly (the absorbance peak shifts from 520 to 650 nm) because their unique localized surface plasmon resonance (LSPR) is changed. These absorbance peak shifts could be identified by collecting them on reflectance signals [19] and then be quantified by using a UV–Vis spectrophotometer [18]. As their color changes are very obvious, it can be easily detected by the naked eye. This kind of aptasensors is more appropriate for their in-situ applications where they can greatly facilitate simple and rapid routine inspections [20]. In this review, we have summarized the performance dependent analysis of almost all of the colorimetric gold nanoparticles-based aptasensors reported till now with a special focus on the detection of antibiotics. Colorimetric aptasensors reported for different antibiotics have been outlined individually with respect to the particular group they belong.
AuNPs diameter is the most important factor in all the reported methods with most of the protocols using the particle size between 10 and 27 nm diameter (most of the methods used 13 or 15 nm) of AuNPs, prepared by classical citrate reduction of HAucl4 or their modifications for example the method used by Huang et al. [21] to prepare AuNPs. Antibiotics have been classified into seven groups on the bases of their functions and molecular structure, including β-lactams (BLCs), aminoglycosides (AGSs), anthracyclines (ACs), chloramphenicol (CAP), fluoroquinolones (FQs), tetracycline (TC) and sulfonamides (SAs).
3. A Generalized Overview, Future Perspective, Challenges and Conclusions
Because the development of innovative or emerging methods for accurate antibiotic detection is an environmental and clinical need, we gave a comprehensive overview of the reported aptasensor advances over the past decade. Some of the aforementioned aptasensors are already being used at commercial level by food industries to detect antibiotic residues for food safety. Some new methods are still in theoretical and early testing phase with a high potential for marketing in the future. Colorimetric aptasensors are highly advantageous as the experimental results can be directly observed/quantified by analyzing visual color changes via naked eye, spectrophotometer or mobile phone chromatism without any kind of complex equipment/instrumentation. Mostly, 5’-end of the different aptamer sequences were used, preferably for immobilization purposes. The specificity of the used aptamer sequences towards their analytes (in many protocols) was verified by testing their sensitivity against possible interfering or structurally similar substances. Nanotechnology made a significant contribution in the field of biosensing; e.g., combining particular aptamer sequences with different nanomaterials led to the development of highly selective and sensitive aptasensors. There are numerous similarities and differences between various sensing protocols as described previously in this review such as, all colorimetric aptasensors discussed here, involves the use of a sensing or recognition material (aptamer) and a colorimetric agent (AuNPs) for signal transduction. From the sensing viewpoint, the inherent properties of AuNPs also offer numerous benefits for the construction of smart sensors, e.g., surface adsorption, dynamic light scattering with the change in their particle size, and the salt-induced aggregation of the AuNPs. However, not all of these aptasensors have the same recognition systems or obey the same working principle (already described for several reports). Subsequently, these kinds of portable devices are simple to operate, rapid, inexpensive, and suitable for on-site detection applications with high sensitivity, selectivity and flexibility. However, some limitations and challenges still need to be addressed here.
All of the protocols used DNA aptamer sequences. RNA sequences should also be characterized for their use in this kind of detection methods. Most of the reported aptasensors are intended to be extended for the detection of a number of different low molecular weight analytes (other antibiotics) by changing the particular aptamer sequence with the most suitable one against the other intended targets to be detected. Unfortunately, not enough high-affinity aptamer sequences have been developed to be replaced for an already existing protocol. We have cited 40 different protocols reported over the decade so far, with most of the studies using the same once-developed aptamer sequence for a specific analyte again and again in different protocols. Despite numerous advances currently made in the field of aptamer based biosensing platform, they are still considered immature if we compare them with immunosensors. This may be attributed to a limited high efficiency aptamer variety available at present. Screening of class specific aptamers to detect common group members can also be helpful to recognize more antibiotic types. Thus, there is an urgent need for more high affinity aptamer sequences to be screened for a wide range of antibiotics (small molecular targets) in the future. Aptamer immobilization approaches, especially their bioconjugation with AuNPs or other nanomaterials, may sometimes confine the aptamer’s target recognition capacity as there is relatively less knowledge regarding the surface-immobilization techniques for aptamers. There is an increasing demand to develop some novel modification and immobilization strategies concerning greater nanomaterials biocompatibility so that the aptamers may attach easily to their functional signal reporters. Because the on-site detection of the antibiotic targets is challenging, it needs to be given more attention. Additionally, environmental or food sample (to be analyzed) conditions, e.g., viscosity, ionic strength, pH, as well as non-specific interactions of the sample matrix with aptamer, must be considered.
Further studies are required to investigate the competing ligand interference, in other words to address the false-negative/ false-positive results or to confirm the selectivity of the aptasensor against their specific target antibiotic. Most importantly, attention is needed to fill the gap of certain parameters (such as pH, temperature, ionic strength, etc.) for the compatibility between aptasensor design and aptamer selection (selection conditions should be same) so as to improve the identification capacity of the aptamer which may indirectly enhance the performance of the sensor in terms of stability, specificity, affinity and sensitivity. Besides this, some external factors may also easily influence the colorimetric assays such as the color of the sample and background fluorescence. Some other challenges are associated with longer fabrication time duration and poor stability or reuse.
By considering the significant advances in the above-mentioned directions, we aim to inspire more efforts for the development of AuNPs based colorimetric aptasensors for on-site detection of antibiotics and other small molecule pollutants. In the end, we would like to recommend improvements by combining various highly efficient AuNPs-based colorimetric aptasensor strategies on one platform to allow multiple residue detections from the only sample as a source. Overall, although there is still a long way to go, we are hopeful that aptamer-based biosensing methods, especially AuNPs calorimetry, will ultimately be proved to be a real-world tool, meeting the present and future challenges which might be impossible otherwise, with the latest available methods. Thus, more efforts are required to manufacture more commercially available aptasensors with high efficiency, easy operation, and low-cost.
Fast and precise assays for the detection of antibiotics still face certain limitations such as sensitivity, specificity, linearity, turnaround time, cost effectiveness, health risks, etc. Nanotechnology is believed to be key to answering these questions while minimizing systemic toxicity. Aptamers offer several advantages over other materials, such as high binding specificity and physicochemical stability, simple modification, long shelf life, minor variation, slight denaturation susceptibility, and cost effectiveness. They interact with a variety of molecules including antibiotics. The enzyme-like activities of gold nanoparticles potentiate their role in biosensing systems and opens the road for their application in various fields of scientific research, diagnostics, and therapy. These superiorities motivate the development of aptasensors for wider applications.
This entry is adapted from the peer-reviewed paper 10.3390/nano11040840
References
- Pfeiffer, F.; Tolle, F.; Rosenthal, M.; Brändle, G.M.; Ewers, J.; Mayer, G. Identification and characterization of nucleobase-modified aptamers by click-SELEX. Nat. Protoc. 2018, 13, 1153–1180.
- Liu, D.; Zhang, Z.; Yin, Y.; Jia, F.; Wu, Q.; Tian, P.; Wang, D. Development and evaluation of a novel in situ target-capture approach for aptamer selection of human noroviruses. Talanta 2019, 193, 199–205.
- Sabri, M.Z.; Hamid, A.A.A.; Hitam, S.M.S.; Rahim, M.Z.A. In-silico selection of aptamer: A review on the revolutionary approach to understand the aptamer design and interaction through computational chemistry. Mater. Today Proc. 2019, 19, 1572–1581.
- Ahirwar, R.; Nahar, S.; Aggarwal, S.; Ramachandran, S.; Maiti, S.; Nahar, P. In silico selection of an aptamer to estrogen receptor alpha using computational docking employing estrogen response elements as aptamer-alike molecules. Sci. Rep. 2016, 6, 21285.
- Song, Y.; Zhu, Z.; An, Y.; Zhang, W.; Zhang, H.; Liu, D.; Yu, C.; Duan, W.; Yang, C.J. Selection of DNA Aptamers against Epithelial Cell Adhesion Molecule for Cancer Cell Imaging and Circulating Tumor Cell Capture. Anal. Chem. 2013, 85, 4141–4149.
- Wolter, O.; Mayer, G. Aptamers as valuable molecular tools in neurosciences. J. Neurosci. 2017, 37, 2517–2523.
- Tomilin, F.N.; Moryachkov, R.; Shchugoreva, I.; Zabluda, V.N.; Peters, G.; Platunov, M.; Spiridonova, V.; Melnichuk, A.; Atrokhova, A.; Zamay, S.S.; et al. Four steps for revealing and adjusting the 3D structure of aptamers in solution by small-angle X-ray scattering and computer simulation. Anal. Bioanal. Chem. 2019, 411, 6723–6732.
- Sun, H.; Zu, Y. A highlight of recent advances in aptamer technology and its application. Molecules 2015, 20, 11959–11980.
- Gao, H.; Tian, Y.; Zhang, M.; Liu, J.; Yuan, Y.; Tan, J.; Ma, A. Selection, identification, and application of aptamers against Agaricus bisporus lectin to establish an aptamer-AuNPs colorimetric method for detection of ABL. J. Food Qual. 2020, 2020.
- Li, L.; Xu, S.; Yan, H.; Li, X.; Yazd, H.S.; Li, X.; Huang, T.; Cui, C.; Jiang, J.; Tan, W. Nucleic acid aptamers for molecular diagnostics and therapeutics: Advances and perspectives. Angew. Chem. Int. Ed. 2020, 60, 2221–2231.
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63.
- Sharma, R.; Ragavan, K.; Thakur, M.; Raghavarao, K. Recent advances in nanoparticle based aptasensors for food contaminants. Biosens. Bioelectron. 2015, 74, 612–627.
- Mohammadpour, A.H.; Tavassoli, A.; Khakzad, M.R.; Zibaee, E.; Afshar, M.; Hashemzaei, M.; Karimi, G. Effect of gold nanoparticles on postoperative peritoneal adhesions in rats. Nanomed. J. 2015, 2, 211–216.
- Babaei, M.; Ganjalikhani, M. A systematic review of gold nanoparticles as novel cancer therapeutics. Nanomed. J. 2014, 1, 211–219.
- Chen, H.; Zhou, K.; Zhao, G. Gold nanoparticles: From synthesis, properties to their potential application as colorimetric sensors in food safety screening. Trends Food Sci. Technol. 2018, 78, 83–94.
- Kim, C.-H.; Lee, L.-P.; Min, J.-R.; Lim, M.-W.; Jeong, S.-H. An indirect competitive assay-based aptasensor for detection of oxytetracycline in milk. Biosens. Bioelectron. 2014, 51, 426–430.
- Xu, C.; Ying, Y.; Ping, J. Colorimetric aggregation assay for kanamycin using gold nanoparticles modified with hairpin DNA probes and hybridization chain reaction-assisted amplification. Microchim. Acta 2019, 186, 448.
- Ma, Q.; Wang, Y.; Jia, J.; Xiang, Y. Colorimetric aptasensors for determination of tobramycin in milk and chicken eggs based on DNA and gold nanoparticles. Food Chem. 2018, 249, 98–103.
- Seo, H.B.; Kwon, Y.S.; Lee, J.E.; Cullen, D.; Noh, H.M.; Gu, M.B. A novel reflectance-based aptasensor using gold nanoparticles for the detection of oxytetracycline. Analyst 2015, 140, 6671–6675.
- Wu, Y.-Y.; Liu, B.-W.; Huang, P.; Wu, F.-Y. A novel colorimetric aptasensor for detection of chloramphenicol based on lanthanum ion–assisted gold nanoparticle aggregation and smartphone imaging. Anal. Bioanal. Chem. 2019, 411, 7511–7518.
- Huang, P.-C.; Gao, N.; Li, J.-F.; Wu, F.-Y. Colorimetric detection of methionine based on anti-aggregation of gold nanoparticles in the presence of melamine. Sens. Actuators B Chem. 2018, 255, 2779–2784.
