Downy mildews affect important crops and cause severe losses in production worldwide. Accurate identification and monitoring of these plant pathogens, especially at early stages of the disease, is fundamental in achieving effective disease control. The rapid development of molecular methods for diagnosis has provided more specific, fast, reliable, sensitive, and portable alternatives for plant pathogen detection and quantification than traditional approaches.
- downy mildews
- molecular diagnostics
- plant pathogens
1. Downy Mildew Pathogens
Downy mildew (DM) pathogens include several species of obligate oomycetes that can cause devastating damage to commercial [1[1]], landscape [2], and natural ecosystem plants [3,4,5]. Species such as Plasmopara viticola [6], Pseudoperonospora cubensis [7], Pseudoperonospora humuli [8,9], Peronospora belbahrii [10], Plasmopara obducens [11], Peronospora tabacina [12] Peronospora effusa [13], Peronosclerospora philippinensis, and Sclerophthora rayssiae var. zeae [14] have resulted in significant losses due to downy mildew epidemics around the world. In some instances, the epidemics have been so severe that they have prompted historical shifts in crop production [15,16,17,18]. In addition to the aggressiveness of these pathogens, fungicide insensitivity further compounds losses attributed to disease [19,20,21,22]. Thus, research to improve diagnostics and management of downy mildew pathogens has become a priority for the scientific community in recent years [23,24,25,26].
2. How to Find Downy Mildew Pathogens
The diagnostics of downy mildew diseases has mainly relied upon direct observation of symptoms and signs using the naked eye or hand lenses and microscopes [27]. This is possible after observing their sexual (e.g., antheridia and oogonia) and asexual structures (e.g., sporangiophores, sporangia, and zoospores) (Figure 1) involved in survival and dispersion, and because many downy mildew pathogens produce distinctive foliar signs and symptoms when colonizing a host plant [2,28,29]. However, such methods fall short when detection in seed or planting material is needed [8,23], when symptoms and/or signs are not characteristic enough, resulting in misdiagnosis [30], or when the pathogen identity to species, pathotype, or clade level has disease management implications [31] (Figure 2).
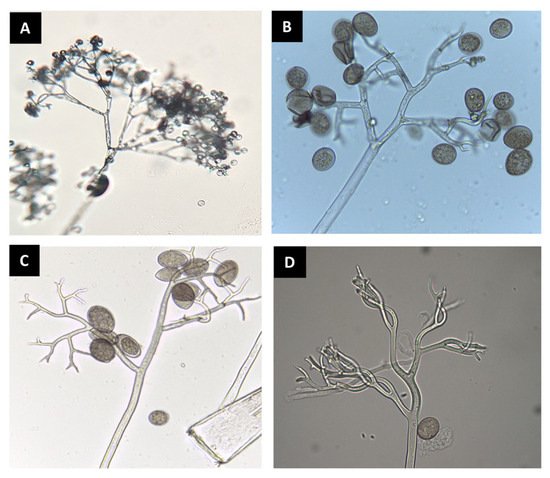
Figure 1. Sporangiophores and sporangia of downy mildew pathogens observed under a compound microscope. Bremia lactucae (A); Peronospora belbahrii (B); Pseudoperonospora cubensis (C); Peronospora chenopodii-ambrosioidis (D).
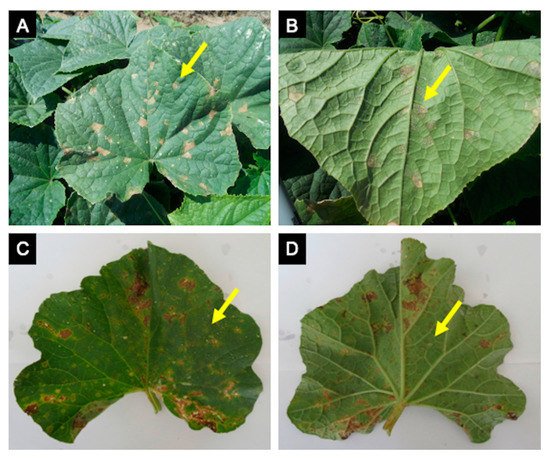
Figure 2. Cucurbit downy mildew caused by Pseudoperonospora cubensis in cucumber and cantaloupe. Cucumber symptoms (A) and signs (B) are very distinct, while cantaloupe symptoms (C) are often confused with other leaf spots or injury due to little sporulation on the underside of the leaf (D).
The rapid identification of disease-causing organisms as well as disease forecasting to reduce the use of pesticides [35,36] have created the necessity to develop more rapid, sensitive, versatile, high-throughput, and cost-efficient markers to identify and quantify plant pathogens. Unfortunately, downy mildew pathogens have been under-represented in oomycete phylogenetic studies and marker development [37]. Visual vs. molecular approaches for downy mildew diagnostics have different advantages and disadvantages [34], but rapid and accurate diagnostics are needed because, under favorable weather conditions, a field or greenhouse infected with downy mildew can result in complete loss of the crop in just a few days [38,39,40]. On-site visual inspection of symptoms and signs may provide a rapid diagnosis but requires trained personnel familiar with the particular downy mildew disease and the presence of distinct symptoms and/or signs [8,27,30]. Molecular diagnostic assays can be performed within a day but, in most cases, require a sample being taken to the laboratory, which can delay the process by several days [34]. This is also true when using conventional microscopy in the laboratory if on-site identification is not possible [41]. Molecular diagnostics can also provide additional information other than pathogen identity, such as fungicide sensitivity, virulence, aggressiveness, or pathogenicity, if such markers are available for a downy mildew pathogen [32,33]. However, for practical use of this information in disease management, results from assays need to be available quickly [41]. In this regard, field-deployable platforms for molecular diagnostics will be critical to unlock the potential for novel molecular markers and technologies to revolutionize disease management [42,43,44].
3. Different Marker Types
Visual-based diagnostic methods for plant pathogens are largely based on the observation of phenotypic characters and host range associations that require time and expertise for interpretation [45] and are frequently limited to taxonomic determination above species. By contrast, molecular techniques have changed the diagnostic emphasis towards pathogen presence and quantity using nucleic acids or proteins [46]. These techniques include serology (immunology), isoenzyme comparison, and nucleic acid-based technologies [44,46,47] (Table 1).
| Source | Loci/Type | Advantages | Disadvantages | Examples |
|---|---|---|---|---|
| Nuclear | Ribosomal and Internal Transcribed Spacer (ITS) | -High reproducibility -Abundant copies -Common primers |
-Copy heterogeneity -Low resolution for cryptic species -Species cross-reactivity |
Pe destructor [70] Pe arborescens [71] Ps belbahrii [72] Plasmopara spp. [64] B.lactucae [64] Ps humuli [73] |
| Housekeeping | -Common primers -known genes |
-Low polymorphisms and reproducibility -Limited for phylogenetic analysis |
Ps belbahrii [26] Ps cubensis [74] |
|
| Species-specific | -species-specific primers |
-Low polymorphisms and reproducibility -Limited for phylogenetic analysis |
Ps humuli [23] Ps cubensis [32,33] |
|
| Multilocus | -Improves phylogenetic interpretation -Infraspecific resolution -High variability |
-Low reproducibility -More labor |
Ps cubensis [66] Ps. humuli [66]. |
|
| Mitochondrial | Single locus | -Improves phylogenetic interpretation -Infraspecific resolution -High variability |
-Uniparental heritance -Limited in detecting hybrid species |
B. lactucae [75] Ps cubensis [76] |
| Antigen | ELISA | -Speed and simplicity -High-throughput |
-Requires monoclonal antibodies -Species cross-reactivity -Limited use for biosurveillance |
Pe destructor [54] |
| Immunostrips | -Speed and simplicity -Cost-effective -Portability |
-Requires monoclonal antibody -Species cross-reactivity |
Still not available for downy mildew pathogens | |
| Enzymatic profile | Isozymes | -Codominant markers -Complement to phenotypic data |
-Large amount of tissue is required -Low polymorphisms -Influenced by the environment |
Pernosclerospora spp. [58] P. halstedii [59] |
The detection and identification of downy mildew pathogens are fundamental for disease management. Molecular diagnostic assays do not have most of the disadvantages associated with traditional detection methods, but the selection of a particular molecular assay is not free of caveats and depends on the main objective (detection, biosurveillance, pathogen relatedness, decision making on fungicide use, pathogen population structure, etc.). However, the development of more accurate DNA-based tests and better molecular markers for the detection of species and infraspecific taxonomic categories will continue as we increase the genetic and genomic information about downy mildew pathogens.
3. Future Prospects
While morphological features of downy mildew pathogens are identifiable by trained experts, using molecular inoculum detection tools represents a diagnostic tool to identify downy mildews with high specificity. The molecular tools available range from expensive technical formats with the ability to quantitate rapidly (e.g., qPCR) to handheld devices for in-field detection (e.g., LAMP, RPA), which give diverse options for finding downy mildews. Furthermore, given the potential for genetic differences informing management decisions (e.g., fungicide sensitivity of species or clades), molecular inoculum detection tools can be used for fungicide timing and selection by non-expert users. Ultimately, the goal of these approaches is to bring the diagnostic tool closer to the point of care with a tool that is simple, quick, and highly accurate that can detect and diagnose diseases. Future research in these areas should allow us to more accurately find these fantastic downy mildews.
This entry is adapted from the peer-reviewed paper 10.3390/plants10030435
References
- Quentin Gascuel; Yves Martinez; Marie-Claude Boniface; Felicity Vear; Magalie Pichon; Laurence Godiard; The sunflower downy mildew pathogenPlasmopara halstedii. Molecular Plant Pathology 2014, 16, 109-122, 10.1111/mpp.12164.
