The liver-heart axis is a growing field of interest owing to rising evidence of complex bidirectional interplay between the two organs. Recent data suggest non-alcoholic fatty liver disease (NAFLD) has a significant, independent association with a wide spectrum of structural and functional cardiac diseases, and seems to worsen cardiovascular disease (CVD) prognosis.
- non-alcoholic fatty liver disease
- fatty liver
- cardiovascular diseases
- ischemic heart disease
- atrial fibrillation
- pathophysiology
1. Introduction
Non-Alcoholic Fatty Liver Disease (NAFLD) is currently thought to cause most cases of chronic liver disease (CLD) worldwide, accounting for as high as 75% of them in some studies [1]. NAFLD is currently recognized as the hepatic clinico-pathological manifestation of metabolic syndrome (MetS)—which also includes abdominal obesity, arterial hypertension, atherogenic dyslipidemia, and reduced insulin sensitivity, all of which were subsequently shown to be closely associated with NAFLD [2,3,4]. The prevalence of NAFLD is on the rise, with variable estimations ranging from 20% to 50% among adults in Western countries [5,6,7]. This rise should not be surprising, due to NAFLD association with MetS, and the current growing epidemics of MetS in the West [8]. Further strengthening the association with MetS, it was shown that NAFLD prevalence rises to 70–75% among individuals with type 2 diabetes (T2DM) and up to 95–99% in those with obesity [9,10]. Although NAFLD was initially identified as a fatty liver disease arising in the absence of significant alcohol intake, it has subsequently been increasingly correlated to a glucose and lipid metabolic derangement. Accordingly, an expert panel recently proposed to change nomenclature from NAFLD to ‘metabolic associated fatty liver disease’ (MAFLD) [11]. The relationship of NAFLD with visceral obesity and alterations in glucose (insulin resistance, diabetes mellitus) and lipid (hypertriglyceridemia, low HDL cholesterol, hypercholesterolemia) [12] metabolism implicates this disease in a large range of clinical conditions relevant not only to liver disease, but also cardiovascular disease (CVD), diabetes, and cancer, making NAFLD/MALFD an emerging topic in clinical medicine.
2. NAFLD Diagnosis: Current Approaches
NAFLD is a broad term comprising a wide spectrum of liver diseases, from simple steatosis or liver fat accumulation—which usually occurs early in the disease course—to more advanced stages of hepatic disease, including non-alcoholic steatohepatitis or NASH, cirrhosis, and liver cancer. Simple steatosis can progress to inflammation and necrosis (i.e., to NASH), and subsequently evolve into cirrhosis and hepatocellular carcinoma [13]. The diagnosis of NAFLD is that of exclusion and requires identification of hepatic steatosis by histology (defined as liver fat infiltration comprising >5% of hepatocytes) or imaging (bright liver echo-pattern), and exclusion of secondary causes of CLD, such as alcohol (<30 g/day for men and <20 g/day for women), congenital or autoimmune diseases, viruses, and hepatotoxic drugs [14].
The diagnosis of NAFLD can be achieved by several techniques. Although liver biopsy and demonstration of steatosis and other histology hallmarks remain the gold standard for NAFLD diagnosis, other less-invasive techniques such as ultrasonography (US), computed tomography (CT), and magnetic resonance imaging are widely utilized clinically because of a better safety profile and availability, lesser cost, and good sensitivity and specificity compared to the gold standard [13,15,16]. Moreover, several European associations, including the European Association for the Study of the Liver, recommend US as the first-line diagnostic procedure for NAFLD in most patients [13]. However, accounting for the limitations of each modality is crucial for proper NAFLD diagnosis. For instance, the sensitivity of US for NAFLD decreases when <30% of hepatocytes are involved, but this modality may also lose sensitivity in morbidly obese individuals [15,16].
Several newer noninvasive approaches have been investigated for the diagnosis of NAFLD, and some of them have been introduced in clinical practice (Table 1).
Table 1. Current strategies to diagnose and stage NAFLD.
| Test | Type | Invasiveness | Accuracy ^ in Steatosis Detection | Liver Fat Detection | Liver Fibrosis Staging | Reference |
|---|---|---|---|---|---|---|
| Liver biopsy | Histology | +++ | >99% | ✓ | ✓ | [17] |
| MRI-PDFF | Imaging | - | 98% | ✓ | ✓ | [18] |
| US | Imaging | - | 91% *, 93% ** | ✓ | ✓ | [19] |
| DGE-MRI | Imaging | + | 83% *, 94% *** | ✓ | ✓ | [20] |
| CT | Imaging | + | 67% *, 90% *** | ✓ | ✓ | [20] |
| US-FLI | Imaging | - | 90% | ✓ | ✕ | [21] |
| Fatty Liver Index | Score based on biochemical parameters | - | 84% | ✓ | ✕ | [22] |
| NAFLD Fat Score | Score based on biochemical parameter | - | 76% | ✓ | ✕ | [23] |
| CAP | Imaging | - | 76% | ✓ | ✕ | [24] |
| Fib-4 | Score based on biochemical parameters | - | 90% | ✕ | ✓ | [25] |
| VCTE | Imaging | - | 89% | ✕ | ✓ | [26] |
| NAFLD Fibrosis Score | Score based on biochemical parameters | - | 86% | ✕ | ✓ | [27] |
| APRI | Score based on biochemical parameters | - | 48%, 66% | ✕ | ✓ | [28] |
APRI, AST to Platelet ratio index; CT, Computed tomography; DGE-MRI, dual gradient echo magnetic resonance imaging; US, Ultrasonography; MRI-PDFF, MRI-based proton-density fat fraction; US-FLI, ultrasonographic fatty liver indicator; VCTE, Vibration-controlled transient elastography; CAP, Fibroscan Controlled Attenuation Parameter. ^ Accuracy was calculated as Accuracy = ((Sensitivity) * (Prevalence)) + ((Specificity) * (1 − Prevalence)) based on data derived from the referenced publications. * detection of ≥5% of steatosis ** detection of ≥10% of steatosis *** detection of ≥30% of steatosis.
The fatty liver index (FLI) [22] is calculated based on levels of triglycerides, body mass index (BMI), waist circumference, and γ-glutamyl-transpeptidase. A FLI < 30 rules out NAFLD whereas a FLI ≥ 60 rules in hepatic steatosis. Scores between 30 and 59 are considered undetermined. The ultrasound fatty liver index (US-FLI) [29] is a different score based on US parameters: a score > 2 suggests the presence of steatosis. Another accurate method to quantify hepatic steatosis is represented by assessment of the controlled attenuation parameter (CAP) on hepatic Fibroscan [30]. In NAFLD, Fibroscan has also been used to assess liver stiffness, a measure of liver fibrosis, by transient elastography (TE). The NAFLD Fat Score is another score that can be used to predict NAFLD and liver fat content (AUROC = 0.88) [23]. It is based on presence of MetS, T2DM, fasting serum (fs) insulin, fs-aspartate aminotransferase (AST), and AST/alanine aminotransferase ratio.
Staging of fibrosis in NAFLD can also be accurately estimated using the NAFLD fibrosis score (NFS) [27] calculated on the combination of several parameters: age, BMI, altered glucose metabolism, AST/ALT ratio, platelet count, and albumin levels. Significant liver fibrosis (equivalent to F3-F4 fibrosis on liver biopsy) is highly suspected when the NFS is >0.675. This measurement needs a diagnosis of NAFLD done with other tests/assays. Two other methods have been used to assess probability of cirrhosis in NAFLD, including the AST to Platelet ratio index (APRI test) [31,32] and the FIB-4 test [25,33].
3. NAFLD as a Systemic Disorder
As numerous compelling evidence shows NAFLD is a systemic disease, rather than confined to the liver, the increasing clinical and economic burden of NAFLD stems from both hepatic and extrahepatic complications. The hepatic complications of NAFLD include NASH, cirrhosis, and hepatocellular carcinoma, and hence it currently represents the second main indication for liver transplantation, projected to become the main one over the next ten years [34,35]. NAFLD affects many other systems and has been shown to increase the risk of developing a number of diseases, including many cardiovascular diseases (CVD), as well as T2DM, chronic kidney disease, and colorectal and other cancers [36,37,38,39,40,41].
4. NAFLD and Cardiovascular Disease: Current Understanding
The relationship between NAFLD and CVD is complex (Figure 1). For instance, NAFLD is associated with many CVDs and the total CVD risk is almost double in patients with NAFLD compared to those without [46]. Moreover, as both conditions share many risk factors, most notably T2DM, dyslipidemia, and obesity, it could be conferred that the same factors account for both diseases and the association being merely a result of these common risk factors [47,48]. However, there is ample evidence showing that a direct mechanistic relationship exists between NAFLD and CVD, and that the former is an independent risk factor for the latter [36,41,42,46,48,49,50]. Due to the association with CVD, recent European clinical practice guidelines mandate screening of the CV system in all patients with NAFLD, at least by detailed risk factor assessment and a comprehensive clinical exam [13].
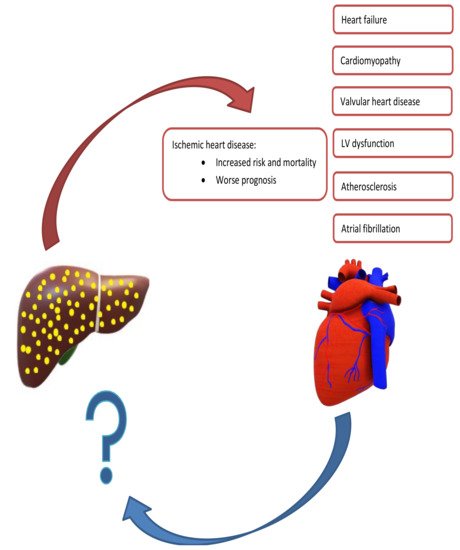
Figure 1. Summary of Mutual Exacerbation of Hepatic and Cardiac Disease Severity. Nonalcoholic fatty liver is associated with several cardiac diseases, including heart failure, cardiomyopathy, ischemic heart disease, and arrythmias. In contrast, there is a paucity of studies evaluating cardiac diseases leading to hepatic dysfunction.
A number of studies suggest NAFLD association with subclinical and clinical CVD, both closely related to atherosclerosis and inflammation. Subclinical CVD is commonly inferred from carotid intima-media thickening (CIMT), increased coronary calcium score (CAC), and abdominal aortic calcification (AAC), whereas clinical CVD includes most commonly ischemic heart disease (IHD) and atrial fibrillation (AF) [41]. In one metanalysis with 3497 subjects, NAFLD was found to be significantly associated with CIMT and NAFLD patients demonstrated a 13% increase in CIMT compared to controls [51]. Similarly, in a large cohort study with 8020 adult men without carotid atherosclerosis (CA) at baseline followed up for eight years, NAFLD regression was associated with a decreased risk of subclinical CA development (HR 0.82) compared to those with persistent NAFLD [52]. The authors explained this association by metabolic factors, which could mediate the effect of NAFLD [52].
Several studies demonstrated an association between NAFLD and CAC, thus implying an increased risk of coronary events in patients with NAFLD [53,54,55,56,57,58,59,60]. In one meta-analysis of 16 cross-sectional studies with 16,433 NAFLD patients and 41,717 controls, it was shown that NAFLD was significantly associated with CAC > 0 and CAC > 100 independent of traditional risk factors [61]. In a second study on 4731 participants with no history of CVD or liver disease and approximately 4 years of follow-up, NAFLD was shown to be associated with CAC development independent of metabolic or CV risk factors [62]. These associations were further established in a recent large meta-analysis on 85,395 participants (including 29,493 patients with NAFLD), where NAFLD was associated with an increased risk of CIMT (OR 1.74), arterial stiffness (OR 1.56), CAC (OR 1.4), and endothelial dysfunction (OR 3.73) [42].
Clinical CVD, most importantly IHD, is the leading cause of death in patients with NAFLD [45,63]. NAFLD patients seem to be at a higher risk of developing IHD [56]. In one study with 445 patients, high risk coronary plaques—assessed by coronary CT angiography—were more likely to occur in patients with NAFLD compared to controls (59.3% vs. 19.0%, respectively) [56]. Similar results were observed in other studies, which concluded higher prevalence of coronary plaques among NAFLD patients independent of other metabolic risk factors [64,65,66]. A striking finding was the observation that higher NAFLD severity directly correlated with higher CVD risk [67]. In one study involving 360 patients with ST-segment elevation myocardial infarction (STEMI), higher NAFLD grade (as assessed by liver US) correlated with higher in-hospital and three-year mortality rates, and the authors concluded recommending NAFLD screening in patients with STEMI [68]. In another study examining 186 non-diabetic patients with STEMI undergone percutaneous coronary intervention (PCI), NAFLD was associated with higher odds of myocardial reperfusion failure, no ST-segment resolution, and higher in hospital major adverse cardiac events (MACE) [69].
AF is the most common sustainable cardiac arrhythmia [36]; the health and economic burden of atrial fibrillation is mainly due to its association with stroke and increased mortality [50]. AF was shown in many studies to be associated with NAFLD [70,71,72,73]. For instance, Targher et al. demonstrated, over a 10-year follow-up, an increased incidence of AF in patients with T2DM and NAFLD compared to patients with T2DM without NAFLD (OR 4.49) [72]. These results were confirmed by a cohort study by Käräjämäki et al. involving 958 hypertensive patients, where they found higher odds of developing AF in patients with NAFLD, independent of T2DM (adjusted OR 1.88) [74].
Several studies suggested NAFLD association with other CVD, including structural and functional cardiac dysfunction and heart valve sclerosis [75]. Some studies reported that NAFLD patients had thicker left ventricular walls during both systole and diastole [76], lower early diastolic relaxation velocity and higher left ventricle (LV) filling pressures [77] and greater LV myocardial mass [78] than patients without NAFLD. In one study assessing cardiac function of 606 T2DM patients according to NAFLD presence, LV diastolic dysfunction was significantly more prevalent in those with NAFLD (59.7% vs. 49%), with stronger association in patients with liver fibrosis independent of insulin resistance and other metabolic risk factors [79]. Moreover, several studies indicated an association between NAFLD and valvular heart disease [80], most commonly aortic valve sclerosis (AVS). In one study with 2212 participants, it was found that patients with NAFLD had 32% higher odds of having AVS than patients without NAFLD, after adjusting for major confounders [81]. This finding was also replicated in T2DM patients with no history of liver or cardiac disease [82,83].
CVDs represent the main cause of mortality in patients with NAFLD, accounting for 40–45% of total deaths, followed by extrahepatic cancers (20%) and liver-related complications (10%) [36,42,43,44,45].
This entry is adapted from the peer-reviewed paper 10.3390/jcm10081569
