The endocannabinoid system (ECS) employs a huge network of molecules (receptors, ligands, and enzymatic machinery molecules) whose interactions with other cellular networks have still not been fully elucidated. Endogenous cannabinoids are molecules with the primary function of control of multiple metabolic pathways. Maintenance of tissue and cellular homeostasis by functional fine-tuning of essential metabolic pathways is one of the key characteristics of the ECS. It is implicated in a variety of physiological and pathological states and an attractive pharmacological target yet to reach its full potential.
- endocannabinoid system
- cancer
- cannabinoid receptor
1. The endocannabinoid system (ECS)
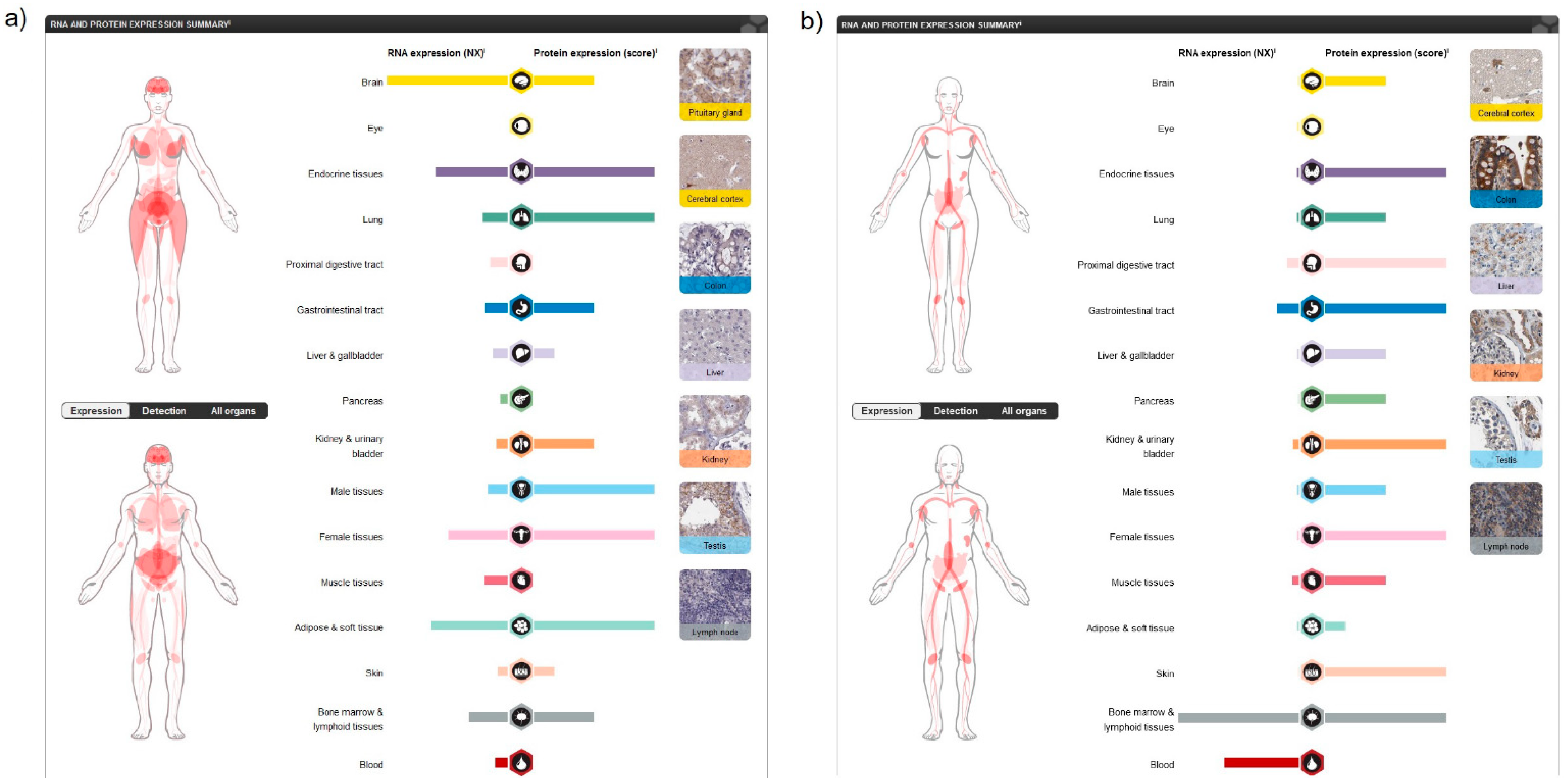
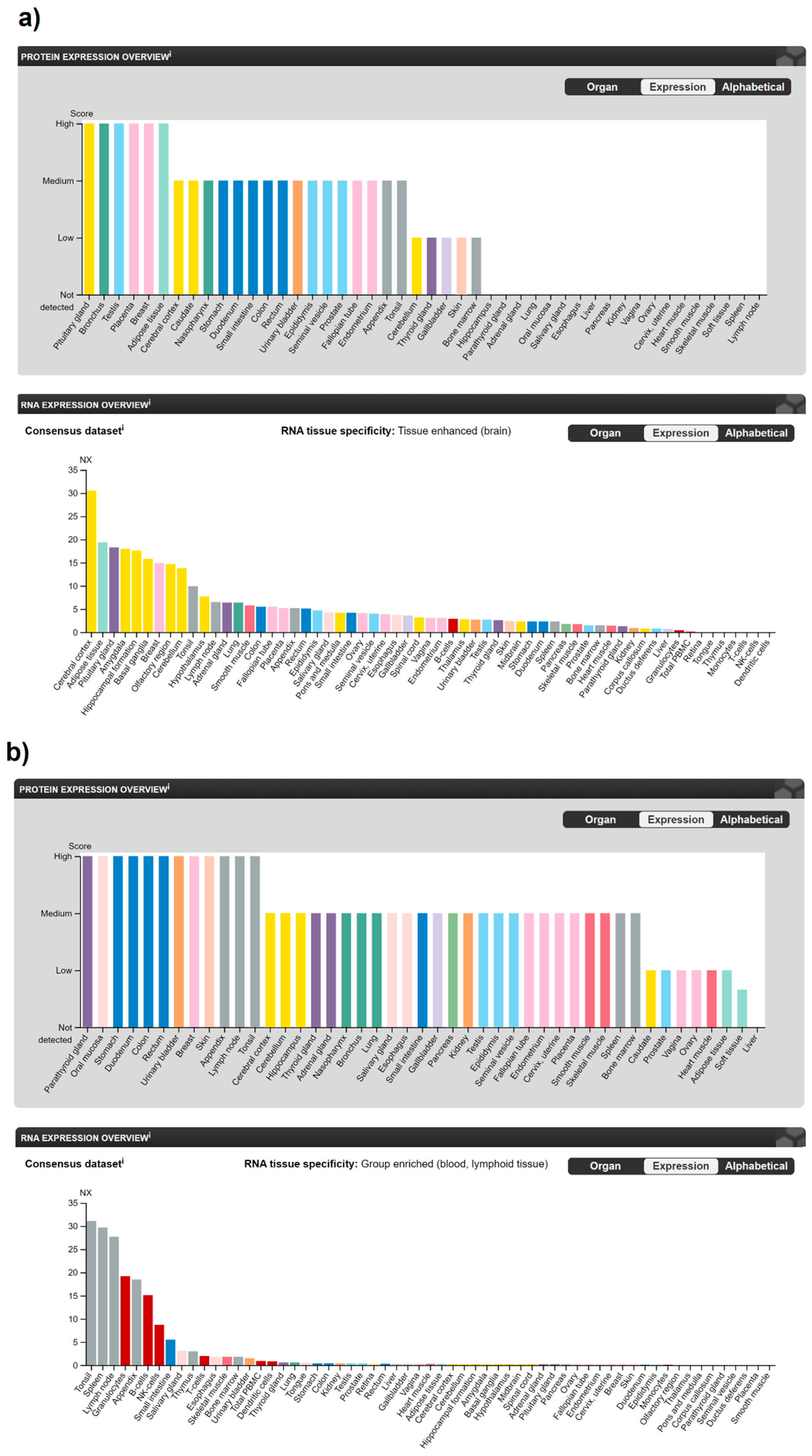
2. Expression of Cannabinoids and Cannabinoid Receptors in Human Cells and Tissues
3. Involvement of the ECS in Specific Physiological and Pathological Processes
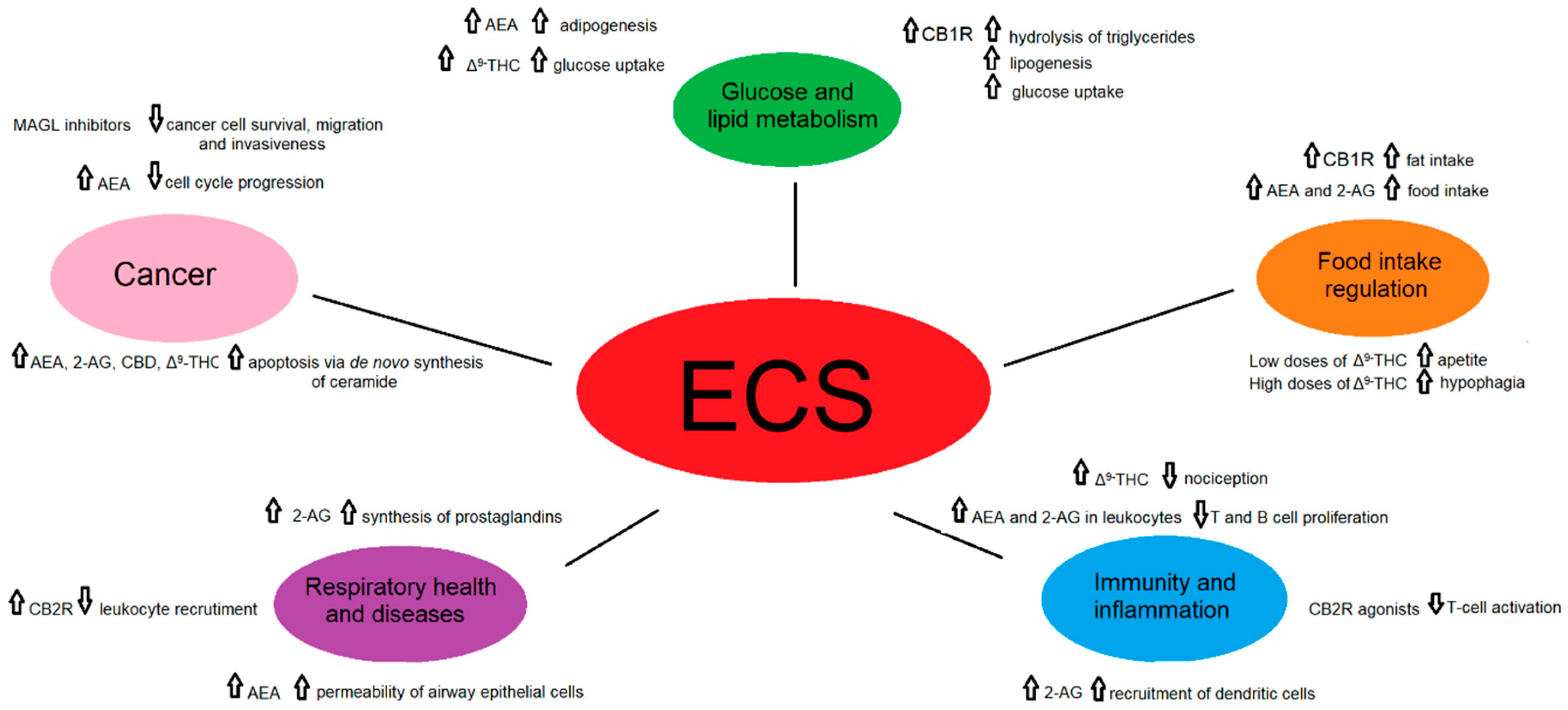
| Process | ECS Component | Metabolic Pathway and/or Effect | Reference |
|---|---|---|---|
| Glucose and lipid metabolism | CB1R stimulation in adipose tissue | increases the activity of the LPL, promotes hydrolysis of triglycerides, favoring lipogenesis through activation of lipogenic enzymes, and inhibition of the activity of the 5′-AMPK and promoting glucose uptake by translocation of the GLUT4 | [22,23,24,25] |
| AEA in adipose tissue | acts as a PPARγ agonist, amplifying the adipogenesis caused by the ECS | [26,27] | |
| CBD blocks CB1R | produces anti-obesity effects and relieves the symptoms of insulin resistance, type 2 diabetes and metabolic syndrome | [28] | |
| Δ9-THC | decreases TAGs and improves glucose uptake by an enhanced GLUT4 and IRS-2 gene expressions | [28,29] | |
| Endocannabinoids in the pancreas | important role in the regulation of cell proliferation and classification of α/β cell during pancreatic islets formation | [30,31] | |
| Activation of hepatic CB1R by endocannabinoids | induces the expression of ACC1, FAS and SREBPF1, resulting in fatty acid synthesis and leading to hepatic steatosis | [32] | |
| JD5037 and AM6545 in genetically and diet-induced obese mice | reduction of obesity, reverse leptin resistance and improve dyslipidemia, hepatic steatosis and insulin resistance and preserve beta cell function | [30,33,34,35] | |
| Food intake regulation | Gastric CB1R activation by fat intake | increases fat-taste perception and promotes fat intake by ghrelin secretion | [30,36] |
| AEA and 2-AG in plasma in humans | acts to initiate the intake and maintains the intake, respectively | [30,37,38,39] | |
| JD5037 in obese mice | diminishes leptinemia and reverses leptin resistance, resulting in a decrease in body weight and food intake | [33] | |
| AM6545 in obese mice | reduces obesity by reversing leptin resistance and improving dyslipidemia, insulin resistance and hepatic steatosis | [30,33,34,35] | |
| Low doses of Δ9-THC | suppression of glutamatergic transmission of CB1R and a rise of appetite | [30,40] | |
| High doses of Δ9-THC | GABAergic transmission of CB1R is disturbed, resulting in hypophagia | [30,40] | |
| Diminished anandamide levels by food enriched in n-3 PUFA | improves the lipid profile in obese subjects, preventing and treating metabolic disorders | [41,42] | |
| Immunity and inflammation | CP55940 acting through CB2R in bone marrow | retention of immature B cells producing a significant decrease in CXCR4 | [43,44,45] |
| 2-AG via activation of CB2R | recruits dendritic cells and their precursors during the innate immune response | [43,46] | |
| Cannabinoids acting through CB2R | inhibition of T-cell activation | [47,48,49] | |
| AEA and 2-AG in leukocytes | anti-inflammatory effects by decreasing T and B cell proliferation and proinflammatory and anti-inflammatory functions, respectively | [50,51] | |
| Rimonabant acting through CB1R | Rimonabant acting through CB1R | [52,53,54,55] | |
| CB2R activation | enhances fat tissue inflammation | [52,56,57] | |
| Increased levels of AEA and 2-AG in the brain with FAAH and MAGL | effective control of the immune response in different models of MS, HD and AD | [52,58] | |
| Δ9-THC in acute, visceral, inflammatory, and chronic pain | reduction of nociception | [59,60] | |
| CBD acting through CBR | inhibition of AEA enzymatic hydrolysis, activation/desensitization of TRPV1 and TRPA1 channels and inhibition ENTs, causing analgesia, and inhibition of inflammation | [60,61] | |
| Respiratory health and diseases | AEA through non-canonical bioactive arachidonic metabolite formation | increases the permeability of airway epithelial cells | [62] |
| 2-AG | source of lung prostaglandins which metabolize into leukotriene B4 and C4 by neutrophils and eosinophils | [63,64] | |
| CB2R activation | inhibition of leukocyte recruitment and secretion of pro-inflammatory cytokines as TNF-α, IL-1β, IL-6, reduction of the formation of reactive oxygen species | [65] | |
| Inhibition of MAGL and FAAH | downregulation of TNF-α, PGE2, COX-2, iNOS | [66,67] | |
| Cancer | AEA through CB1R and CB2R-CXCR4 heteromers in breast cancer | cell cycle progression blocking, inhibition of chemotaxis | [68,69,70] |
| AEA, 2-AG, CBD, Δ9-THC in glioma | induction of apoptosis via de novo synthesis of ceramide, inhibition of cell migration and invasiveness through CB1R and CB2R activation | [71,72,73,74,75] | |
| AEA, 2-AG, Δ9-THC, CBD, HU-331, CP 55,940 acting through CB1R, CB2R and PPARγ in gastrointestinal tumors | cell invasiveness through the AKT/GSK3β signaling axis, induction of apoptosis through the inhibition of RAS–MAPK, PI3K–AKT and increased ceramide synthesis | [76,77,78,79] | |
| phyto-, endo- and synthetic cannabinoids, and MAGL inhibitors acting through CB1R, CB2R and CB2R-CXCR4 in prostate cancer | inhibition of cancer cell survival, migration and invasiveness through adenylyl cyclase, protein kinase A, EGFR | [69,80,81,82] | |
| CBD and CBR over-expression in lung cancer | activation of apoptosis, inhibition of ERK, PI3K, p38 MAPK, Akt, EGFR and ceramide-related pathways, tumor suppression by regulation of angiogenesis through up-regulation of PPAR-γ and cyclooxygenase-2 | [83,84,85] |
3.1. Glucose and Lipid Metabolism
3.2. Food Intake Regulation
3.3. Immunity and Inflammation
3.4. Respiratory Health and Diseases
3.5. Cancer
This entry is adapted from the peer-reviewed paper 10.3390/ijms22073661
