Licorice is one of the oldest used herbs for medicinal purposes and consists of up to 300 active compounds. The main active constituent of licorice is the prodrug glycyrrhizin, which is successively converted to 3β-monoglucuronyl-18β-glycyrrhetinic acid (3MGA) and 18β-glycyrrhetinic acid (GA) in the intestines. Despite many reported health benefits, 3MGA and GA inhibit the 11-β-hydrogenase type II enzyme (11β-HSD2) oxidizing cortisol to cortisone. Through activation of mineralocorticoid receptors, high cortisol levels induce a mild form of apparent mineralocorticoid excess in the kidney and increase systemic vascular resistance. Continuous inhibition of 11β-HSD2 related to excess licorice consumption will create a state of hypernatremia, hypokalemia and increased fluid volume.
- licorice
- glycyrrhizin
- glycyrrhetinic acid
- glabridin
- 11-β-dehydrogenase isozyme 2
- hyperaldosteronism
- hypokalemia
- hypertension
1. Introduction
Licorice is the root of the legume Glycyrrhiza glabra (Figure 1a) that grows in varieties in warm areas like the Middle East, Asia and Southern Europe. It is one of the oldest used herbs in ancient medicine and referred to as “the father of herbal medicine” [1]. Since the 18th century, the primary use comprises mainly licorice extracts (in pharmacy called Succus liquiritae) as a flavoring additive in tea, tobacco, candy (Figure 1b) and other sweets, but the licorice root itself (Liquiritae radix) is still used as a dietary supplement in some parts of the world [7]. Among people preferring alternative or complementary medicine, historical uses for licorice were revived and are still practiced today [8,9,10].
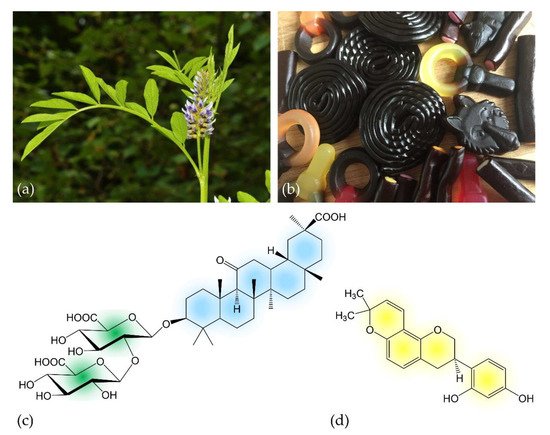
Figure 1. (a) Inflorescence of Glycyrrhiza glabra L.; (b) licorice-containing candies; (c) chemical structure of the prodrug glycyrrhizin (C42H62O16), the main active compound of licorice. The molecule consists of two molecules of glucuronic acid (left) that are linked to 18β-glycyrrhetinic acid; (d) chemical structure of glabridin (C20H20O4), a further bioactive licorice compound. Colors indicate molecule structures used in following schematics.
On the one hand, the health benefits ascribed to licorice are numerous: for centuries it has been used in herbal and folk medicine to treat multiple diseases such as gastrointestinal symptoms and respiratory diseases [10]. The broad spectrum of activities known today comprises immunostimulatory and anti-ulcer effects [11,12,13], anti-viral and anti-microbial effects [14,15], hepatoprotective [16,17], anti-carcinogenic [18] and several other positive effects that contribute to the protection of the nervous, respiratory, endocrine and cardiovascular system [9]. On the other hand, it is well-known that consuming excessive quantities of licorice can impact upon cardiometabolic health by elevating blood pressure (BP), and thus, may be a cause of hypertension and other cardiovascular complications [24,25,26,27,28,29,30].
2. Pharmacological Effects of Licorice
2.1. Licorice Digestion and Chemistry of Metabolites
In the licorice root, tribasic glycyrrhizin naturally occurs in form of its calcium and potassium salts. After oral ingestion, glycyrrhizin (which itself possesses only poor oral bioavailability) is successively hydrolyzed to 3β-monoglucuronyl-18β-glycyrrhetinic acid (3MGA) and the aglycone 18β-glycyrrhetinic acid (GA; also known as enoxolone) by intestinal bacteria possessing specialized β-glucuronidases [36,37]. GA is often considered as the active metabolite of licorice [38,39,40], but its pharmacokinetics seem to be more complex. After rapid absorption from the gut, 3MGA and GA circulate in the bloodstream. From there, they are transported to the liver by carrier molecules, where they are metabolized (Figure 2). In humans, hepatic processing is not yet clearly defined, but it is apparent that each metabolite can undergo further conjugation or reduction followed by biliary excretion [6]. The products are likely re-metabolized by the gut microbiome and thereby subjected to enterohepatic recycling requiring several days for complete elimination [41].
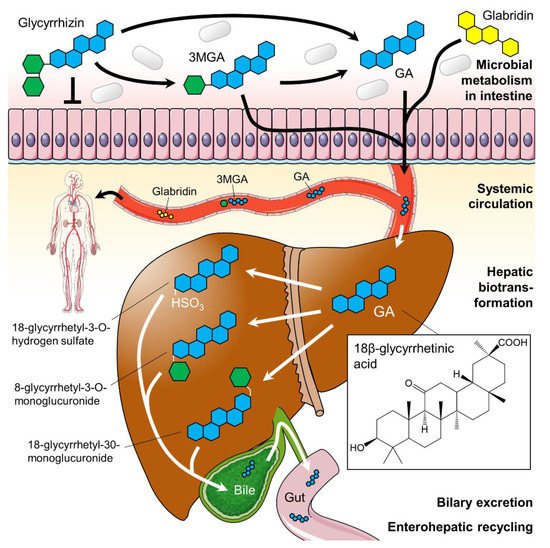
Figure 2. Suggested glycyrrhizin metabolism. Dependent on the gut microbiome glycyrrhizin is stepwise hydrolyzed to 3β-monoglucuronyl-18β-glycyrrhetinic acid (3MGA) and 18β-glycyrrhetinic acid (GA; blue structure) in the intestines. Both 3MGA and GA were absorbed from the gut and transported systemically in the bloodstream. In the liver, they undergo hepatic biotransformation before products were excreted via bile. The flavonoid glabridin (yellow structure) is also absorbed from the gut and circulates in the blood in its aglycone form. The hepatic metabolization of glabridin is not shown here. Green hexagons: glucuronic acid. Parts of the figure were drawn by using pictures from Servier Medical Art (http://smart.servier.com), licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0).
The digestion of licorice is still not completely understood. Interestingly, the bioavailability of glycyrrhizin is reduced when consumed as licorice [45], suggesting that some components of the licorice root may interact with glycyrrhizin during intestinal absorption, reducing its oral bioavailability [46].
2.2. Pharmacodynamics of Licorice Constituents and Metabolites
Licorice intake induces physiological effects similar to aldosterone and corticosteroids. Resembling steroid-like structures, both 3MGA and GA are able to bind to the mineralocorticoid receptor (MR) in the distal tubules of the kidney (direct effect), although competitive binding assays revealed that the affinities of MR for licorice metabolites were up to 10,000 times weaker than those for adrenocortical hormones [49]. In a normal physiological state, MR is activated by aldosterone to increase sodium and water resorption into the blood and potassium excretion into the urine mediating sodium and water homeostasis within the kidneys. However, it is unclear how the direct effects of 3MGA and GA on MR contribute to the effect of licorice. Although there is some evidence of this direct effect in vitro [50], the relative affinity for MR compared to aldosterone as well as low serum levels of GA after licorice consumption, which did not reach the concentrations necessary to affect aldosterone or cortisol binding to MRs in humans, question that theory [51]. In adult tissues, the type II isozyme of 11β-hydroxysteroid dehydrogenase (11β-HSD2) is expressed in the distal nephron of the kidney [54], in smooth muscle cells and endothelial cells of the vascular wall [55], in the heart [56] and in the brain [57], where it serves to protect the MR from being overly activated by cortisol [53,58]. 11β-HSD2 converts ‘active’ cortisol to the ‘inactive’ cortisone which has a very low affinity for MR. Kato et al. [61] suggested that 3MGA, not GA, is the mainly causative agent of licorice-induced pseudohyperaldosteronism. In the kidneys, 11β-HSD2 inhibition by 3MGA or GA (Ki: 5–10 nM) results in a significant increase of active cortisol concentration in the renal tissue leading to a syndrome of apparent mineralocorticoid excess (Figure 3a) [52,62]. In the vascular wall, it increases arterial tone enhancing contractile responses to pressor hormones and reducing endothelial nitric oxide production [57,63].
A vasorelaxant effect of glabridin was described in rat mesenteric arteries, which was associated with the opening of potassium channels and a concomitant rise in tissue cyclic guanosine monophosphate levels [65].
Taken together, intake of licorice induces a mild form of apparent mineralocorticoid excess causing MRs to be activated by both cortisol and aldosterone via inhibition of enzymes necessary for their catabolism (Figure 3). The direct effects of 3MGA and GA on MRs seem to be only negligible in physiological conditions. In the kidney, MR activation leads to transcription of epithelial sodium channel (ENaC), Na+/K+ ATPase and mitochondrial enzymes, which accelerate adenosine triphosphate (ATP)-production (Figure 3b). The final consequences comprise elevated BP, sodium and water retention, decreased plasma potassium (hypokalemia) and caused a suppression of plasma renin and aldosterone levels [66]. In vascular smooth muscle cells, MR activation may further cause vascular stiffening by remodeling of the vascular wall [67].
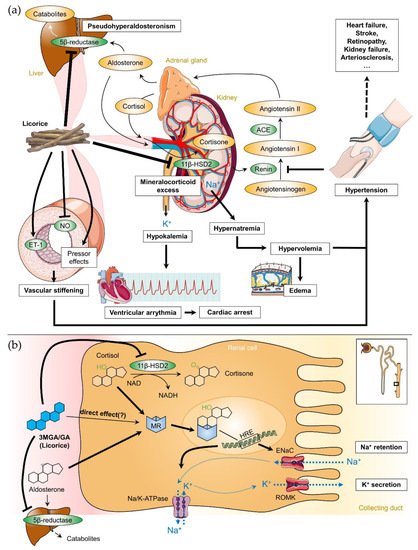
Figure 3. (a) Correlation between licorice intake, the renin-angiotensin-aldosterone-system and licorice-induced adverse effects on the cardiovascular system. (b) Detailed pharmacodynamics of 3β-monoglucuronyl-18β-glycyrrhetinic acid (3MGA) and 18β-glycyrrhetinic acid (GA; blue structure) in the kidney. In addition to a possible direct binding to the mineralocorticoid receptor (MR), 3MGA and GA have inhibiting effects on 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) and 5β-reductase. 11β-HSD2 converts cortisol to cortisone; 5β-reductase is involved in the degradation of aldosterone in the liver. Inhibition of both enzymes contributes to apparent mineralocorticoid excess. The insert shows the localization of the processes within the Henle loop. ACE: angiotensin converting enzyme, ENaC: epithelial sodium channel, ET-1: endothelin 1, HRE: hormone response element, NAD(H): nicotinamide adenine dinucleotide, NO: nitric oxide, ROMK: renal outer medullary potassium channel. Parts of the figure were drawn by using pictures from Servier Medical Art (http://smart.servier.com), licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0).
2.3. Licorice-Induced Hypertension
Licorice mediates its effect on BP primarily via the inhibition of renal 11β-HSD2 by 3MGA and GA (Figure 3a). Water and sodium retention in the kidney increase the blood volume and elevate BP [5]. The body countermeasures with a refractory lowering of the renin secretion in the kidneys, followed by decreased aldosterone production in the adrenal cortex via angiotensin II. However, the increasing level of cortisol (together with unrestricted activation of MR by cortisol) causes pseudohyperaldosteronism. This in turn results in further increasing blood volume and preload of the heart, thereby raising the mean arterial pressure. Furthermore, GA mediates the development of hypertension via decreased bioavailability of NO and activation of the vascular endothelin (ET-1) system (Figure 3a) which was accompanied by impaired endothelium-dependent relaxation in rats [69]. Activation of the endothelin system was also observed in human hypertension [70], and there is some evidence that increased ET-1 may be related to hypertensive end-organ damage and remodeling [71]. Interestingly, an infusion of GA into the rat brain elevated BP without affecting renal sodium and water resorption [72]. This finding indicated a central hypertensinogenic effect of licorice and suggested a more complex regulation of licorice-induced hypertension beyond the inhibition of 11β-HSD2.
2.4. Cardiovascular Effects of Licorice
Licorice traditionally has been prescribed for treatment of cardiovascular disorders, but its effects are not just benign. From the cardiovascular complication described in the literature, cardiac arrhythmias are the most serious side effect caused by licorice intake due to severe hypokalemia (Figure 3a) [105]. The depletion of the body’s potassium stores can cause a prolongation of the QT interval, which is closely connected with ventricular arrhythmias and tachycardia [106]. As a consequence, several patients experienced a cardiac arrest with a subsequent recovery [107,108,109]. In rats, cardioprotective effects of licorice and its metabolites were observed, which are mostly related to their antioxidant properties. Thirty days of licorice intake improved cardiac function and preserved histology of cardiomyocytes either by augmentation of endogenous antioxidants or by reduction in oxidative stress. Thus, licorice may delay the progression of ischemic heart disease [113]. Some studies suggested that the flavonoid glabridin may also have beneficial effects on the cardiovascular system. The effects described comprise inhibition of low density lipoprotein oxidation and atherogenesis [116], a possible inhibition of NADPH oxidase or an increase in the expression of antioxidant enzymes observed in macrophages [117].
2.5. Interaction of Licorice with Prescribed Drugs
Licorice can interfere with cardiac medications, e.g., with drugs used in the treatment of hypertension such as angiotensin converting enzyme (ACE)-inhibitors [121]. Some licorice compounds including glabridin and GA can interact with other drugs and the human liver microsomal cytochromes P450 and P450 3A4 (CYP3A4) systems [122,123,124,125]. For the pharmaceutical use of licorice, it is important to know and consider the interaction between licorice and drugs metabolized by CYP3A4 and P-glycoprotein.
Licorice decreases the bioavailability of cyclosporine and is thus contraindicated [120] in conjunction with this drug [128]. The intake of licorice should be done with caution, when using antihypertensive drugs. ACE-inhibitors, e.g., captopril, inhibit the angiotensin converting enzyme, limiting levels of angiotensin and aldosterone. It was shown that ACE-inhibitors enhance the effects of 11β-HSD2 which may contribute to the natriuretic effect [131]. Hypokalemia is one of the most serious adverse effects of licorice intake and should be completely avoided with loop-diuretics and thiazides since it can lead to serious hypokalemia and hospitalization [132].
Heck et al. [129] described a toxic effect potentiation of warfarin, a cardiac drug that requires strict dosage adjustment, due to the inhibition of the hepatic microsomal enzymes by licorice.
2.6. Contraindications and Effects of Licorice Overconsumption
In general, people aged over 40, patients with history of cardiac disease or more susceptible to cardiac arrhythmias should avoid excess licorice intake in order to obviate arrhythmias or cardiac arrest caused by licorice-induced hypokalemia. One study investigated patients treated with traditional Japanese medicine containing licorice [135]. They discovered that 24.2% of the patients treated with this medicine developed hypokalemia 34 days after administration. Hypokalemia is a serious state that increases the risk of arrhythmia and is associated with an up to 10-time increase in all-cause mortality [74]. The meta-analysis by Penninkilampi et al. [74] summarized other side effects including rhabdomyolysis, paralysis, hypertensive encephalopathy and cardiac arrest. That is why patients who are on medicines lowering potassium levels (such as thiazide or loop diuretics) should also minimize their licorice intake. The same applies for patients suffering from diarrhea or alcoholism, which can worsen hypokalemia. Licorice can be dangerous in patients treated with antihypertensive drugs such as ACE-inhibitors and diuretics. Due to the salt-retaining effect of 3MGA and GA, people suffering from congestive heart failure or resistant hypertension should completely abstain from products containing licorice. This is also advisable for patients taking digoxin or warfarin to avoid the risk of toxicity. Since 3MGA and GA are known to inhibit 11β-HSD2, licorice ingestion during pregnancy should be avoided. GA consumption impaired the development of the respiratory systems in rats because the conversion of cortisone into cortisol plays an important role in lung maturation [136].
This entry is adapted from the peer-reviewed paper 10.3390/foods8100495
