Glycosphingolipids (GSLs) are composed of a mono-, di-, or oligosaccharide and a ceramide and function as constituents of cell membranes. Various molecular species of GSLs have been identified in mammalian cells due to differences in the structures of oligosaccharides. The oligosaccharide structure can vary depending on cell lineage, differentiation stage, and pathology; this property can be used as a cell identification marker.
- glycosphingolipids
- immune response
- anti-glycan antibody
- very-long-chain fatty acid
- glycoprotein
- tumor-associated antigen
- carcinoembryonic antigen
- cancer-associated antigen
1. Introduction
Glycosphingolipids (GSLs) are cell membrane components composed of a mono-, di-, or oligosaccharide and a ceramide. Various molecular species defined by differences in the oligosaccharide structure have been identified in mammalian cells/tissues [1]. These differences in the oligosaccharide structure were found to be indicative of cell lineage, differentiation status, and certain pathologic processes. Due to these function-related differences in oligosaccharide structure, GSLs can be used as cell identification markers. Indeed, several anti-GSL antibodies that specifically recognize certain glycan structures of GSLs have contributed to stem cell research and the diagnosis of various cancers [2].
Pioneering studies in this area revealed that GSLs are immunogenic substances that function as blood group [3] and cancer-associated antigens [2]. A number of antibodies isolated from animals immunized with cancer cells were shown to react specifically with GSLs expressed by those cells, leading researchers to recognize that GSLs are immunogenic substances. Recent studies also revealed that the immunoglobulin repertoire of healthy humans contains various anti-GSL antibodies [4][5], indicating that endogenous GSLs function as antigens and induce B cells to produce antibodies. Although it is poorly understood why mammalian immune cells produce antibodies against GSLs, which then become self-antigens, a number of antibodies that recognize GSLs have been isolated by exploiting this property.
Several immunization methods have been developed in order to efficiently generate anti-GSL antibodies [6][7][8]. However, as these methods do not enable extensive control of the properties of the antibodies induced, such as their epitope affinity and specificity and class/subclass, there is considerable room for significant improvement in anti-GSL antibody technology. A deeper understanding of the mechanism of GSL recognition by the mammalian immune system is needed for the development of high-performance anti-GSL antibodies and the future use of these antibodies as pharmaceuticals.
2. Structures and Cell Type/Tissue Distributions of Molecular Species of Mammalian GSLs
Figure 1 shows the general structure of mammalian GSLs, and Table 1 shows major examples of GSLs identified as cell-surface antigens.
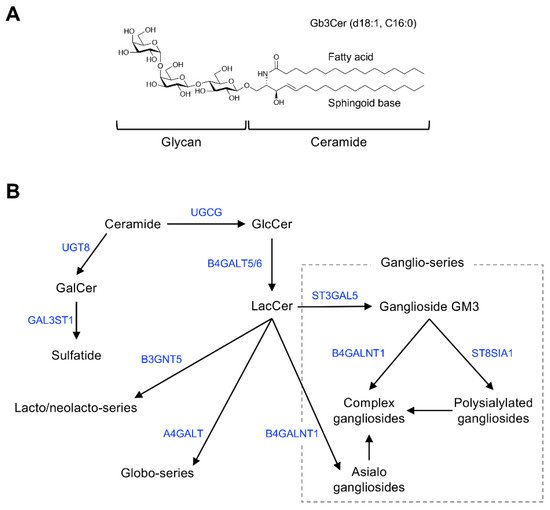
Figure 1. Chemical structure and mammalian glycosphingolipid (GSL) biosynthetic pathway. (A) Chemical structure of a typical mammalian GSL, globotriaosylceramide (Gb3Cer), shown as an example. (B) Arrows and blue font indicate biosynthetic pathway and catalytic enzymes, respectively. Abbreviations: GlcCer, Glcβ1,1Cer; GalCer, Galβ1,1Cer; LacCer, Galβ1,4Glcβ1,1Cer; GM3, Siaα2,3Galβ1,4Glcβ1,1Cer; UGCG, UDP-glucose ceramide glucosyltransferase; UGT8, UDP glycosyltransferase 8; GAL3ST1, galactose-3-O-sulfotransferase 1; B4GALT5/6, β-1,4-galactosyltransferase 5/6; B3GNT5, UDP-GlcNAc: βGal β-1,3-N-acetylglucosaminyltransferase 5; A4GALT, α-1,4-galactosyltransferase; B4GALNT1, β-1,4-N-acetyl-galactosaminyltransferase 1; ST3GAL5, ST3 β-galactoside α-2,3-sialyltransferase 5; ST8SIA1, ST8 α-N-acetyl-neuraminide α-2,8-sialyltransferase 1.
Table 1. Glycosphingolipid (GSL) markers useful for cell identification.
| GSL/Antigen Structure |
Major Expressing Cell or Expressing Cancers | Ref. |
|---|---|---|
| Gb3Cer/CD77 Galα1,4Galβ1,4Glcβ1,1Cer |
Burkitt lymphoma | [9] |
| Gb4Cer/blood group P antigen GalNAcβ1,3Galα1,4Galβ1,4Glcβ1,1Cer |
Erythrocytes | [10] |
| Gb5Cer/SSEA-3 Galβ1,3GalNAcβ1,3Galα1,4Galβ1,4Glcβ1,1Cer |
Stem cells/iPS cells | [11] |
| Sialyl-Gb5Cer/SSEA-4 Siaα2,3Galβ1,3GalNAcβ1,3Galα1,4Galβ1,4Glcβ1,1Cer |
Stem cells/iPS cells | [11] |
| Fucosyl-Gb5Cer/Globo-H Fucα1,2Galβ1,3GalNAcβ1,3Galα1,4Galβ1,4Glcβ1,1Cer |
Breast and other cancers | [12][13] |
| Sialyl Lewisa/CA19-9-terminated GSL Siaα2,3Galβ1,3(Fucα1,4)GlcNAcβ1,3Galβ1,4Glcβ1,1Cer |
Gastrointestinal cancer | [14][15] |
| GD3 Siaα2,8Siaα2,3Galβ1,4Glcβ1,1Cer |
Melanoma | [16] |
| Gg4Cer (asialo-GM1) Galβ1,3GalNAcβ1,4Galβ1,4Glcβ1,1Cer |
Natural killer cells | [17][18] |
Major GSL antigens used to identify human cells are shown. Abbreviations: Cer, ceramide; Fuc, fucose; Gal, galactose; GalNAc, N-acetylgalactosamine; Glc, glucose; GlcNAc, N-acetylglucosamine; Sia, sialic acid.
GSLs are glycoconjugates in which a ceramide (N-acylsphingosine) is glycosidically bound to a mono-, di-, or oligosaccharide glycan. The glycan moiety is located outside of the plasma membrane of the cell due to its hydrophilicity.
The molecular species of GSLs are classified based on their glycan structure [19]. For example, GSLs containing sialic acid are defined as gangliosides. Ceramide structures are also diverse, and the expression of particular GSL molecular species of ceramides is cell type and tissue specific. Monosialoganglioside GM3 (Figure 1B), the principal initiation structure of gangliosides of the ganglio-series, is widely expressed in mammalian cells and tissues. Complex gangliosides, which are synthesized by elongation of the glycan in GM3, are found primarily in nervous system tissues [19][20]. Galactosylceramide (GalCer/cerebroside) and sulfated GalCer (sulfatide) are also abundantly expressed in nervous system tissues as components of myelin [21].
Globo-series GSLs are expressed primarily in blood cells/vessels and the kidney, lung, and intestine, but their abundance is low in nervous system tissues [22][23]. Stage-specific embryonic antigen (SSEA)-3 and -4, expressed specifically in undifferentiated cells such as induced pluripotent stem cells, are also globo-series GSLs (Gb5Cer and sialyl-Gb5Cer, respectively) [11]. GSLs containing sialyl-LewisX, sialyl-Lewisa, and VIM-2 glycans are classified as lacto/neolacto-series GSLs [24][25]. These glycans function as adhesion molecules on the surface of lymphocytes or cancer cells [24][25][26]. The lacto/neolacto-series GSLs expressed in human erythrocytes are blood group substances composed of ABO(H) blood group–type glycans [3][27]. Globo-series GSLs, also known as P blood group substances, are the major GSLs in human erythrocytes [28][10]. Rodent natural killer cells, which specifically express asialo-GM1, can be eliminated by administering anti–asialo-GM1 antibodies to the animals [17][18].
The molecular species of GSLs expressed in mammalian cells vary according to cell differentiation status and pathologic processes. Undifferentiated cells such as stem cells specifically express globo-series GSLs such as SSEA-3 and SSEA-4, whereas these GSLs disappear during differentiation of the cells into somatic cells, which then express other type of GSLs [11]. The type of GSL expressed in a given type of cell or tissues can also differ depending on species. For example, murine stem cells express SSEA-1 (a GSL containing LewisX glycan) [29][30], whereas this GSL antigen is not expressed by human stem cells [11].
Malignant transformation of cells is associated with structural alterations in GSL glycans; cancer cells express different GSLs than their parent cells [2]. For example, disialylated ganglioside GD3 is expressed in melanoma cells [16], Gb3Cer/CD77 is expressed in Burkitt lymphoma cells [9], and globo-H is expressed in breast cancer cells [12][13]. Thus, these GSLs can be monitored as cancer-specific antigens.
A monoclonal antibody that recognizes sialyl-Lewisa as the epitope, NS19-9, was generated by immunizing mice with SW1116 colorectal cancer cells, which specifically express GSLs with sialyl-Lewisa glycan [14]. The NS19-9 antibody can be used to detect a serum glycoprotein containing sialyl-Lewisa, which has been identified as a useful diagnostic marker (CA19-9) for gastrointestinal cancers [15]. In addition to NS19-9, a number of monoclonal antibodies that specifically recognize certain GSL glycan structures as epitopes have been generated by immunizing host animals with various types of cancer cells. These studies led researchers to realize that GSLs are immunogenic substances.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22073776
References
- Hakomori, S. Structure and function of glycosphingolipids and sphingolipids: Recollections and future trends. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 2008, 1780, 325–346.
- Hakomori, S. Tumor-Associated Carbohydrate Antigens Defining Tumor Malignancy: Basis for Development of Anti-Cancer Vaccines. Adv. Exp. Med. Biol. 2001, 491, 369–402.
- Hakomori, S. Blood group ABH and Ii antigens of human erythrocytes: Chemistry, polymorphism, and their developmental change. Semin. Hematol. 1981, 18, 39–62.
- Schneider, C.; Smith, D.F.; Cummings, R.D.; Boligan, K.F.; Hamilton, R.G.; Bochner, B.S.; Miescher, S.; Simon, H.U.; Pashov, A.; Vassilev, T.; et al. The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci. Transl. Med. 2015, 7, 269ra1.
- Luetscher, R.N.D.; McKitrick, T.R.; Gao, C.; Mehta, A.Y.; McQuillan, A.M.; Kardish, R.; Boligan, K.F.; Song, X.; Lu, L.; Heimburg-Molinaro, J.; et al. Unique repertoire of anti-carbohydrate antibodies in individual human serum. Sci. Rep. 2020, 10, 1–15.
- Young, W.W.; Macdonald, E.M.; Nowinski, R.C.; I Hakomori, S. Production of monoclonal antibodies specific for two distinct steric portions of the glycolipid ganglio-N-triosylceramide (asialo GM2). J. Exp. Med. 1979, 150, 1008–1019.
- Brodin, T.; Thurin, J.; Strömberg, N.; Karlsson, K.A.; Sjögren, H.O. Production of oligosaccharide-binding monoclonal antibodies of diverse specificities by immunization with purified tumor-associated glycolipids inserted into liposomes with lipid A. Eur. J. Immunol. 1986, 16, 951–956.
- Okuda, T. Design of Carrier Molecules Suitable for Inducing Immunity to Oligosaccharide Antigens: Application to Anti-Glycoprotein Monoclonal Antibodies. Trends Glycosci. Glycotechnol. 2018, 30, E113–E116.
- Mangeney, M.; Richard, Y.; Coulaud, D.; Tursz, T.; Wiels, J. CD77: An antigen of germinal center B cells entering apoptosis. Eur. J. Immunol. 1991, 21, 1131–1140.
- Yamakawa, T.; Yokoyama, S.; Kiso, N. Structure of Main Globoside of Human Erythrocytes. J. Biochem. 1962, 52, 228–229.
- Handa, K.; Hakomori, S. Changes of glycoconjugate expression profiles during early development. Glycoconj. J. 2016, 34, 693–699.
- Bremer, E.G.; Levery, S.B.; Sonnino, S.; Ghidoni, R.; Canevari, S.; Kannagi, R.; Hakomori, S. Characterization of a glycosphingolipid antigen defined by the monoclonal antibody MBr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J. Biol. Chem. 1984, 259, 14773–14777.
- Mènard, S.; Tagliabue, E.; Canevari, S.; Fossati, G.; I Colnaghi, M. Generation of monoclonal antibodies reacting with normal and cancer cells of human breast. Cancer Res. 1983, 43, 1295–1300.
- Magnani, J.L.; Brockhaus, M.; Smith, D.F.; Ginsburg, V.; Blaszczyk, M.; Mitchell, K.F.; Steplewski, Z.; Koprowski, H. A monosialoganglioside is a monoclonal antibody-defined antigen of colon carcinoma. Science 1981, 212, 55–56.
- Koprowski, H.; Herlyn, M.; Steplewski, Z.; Sears, H.F. Specific antigen in serum of patients with colon carcinoma. Science 1981, 212, 53–55.
- Pukel, C.S.; O Lloyd, K.; Travassos, L.R.; Dippold, W.G.; Oettgen, H.F.; Old, L.J. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J. Exp. Med. 1982, 155, 1133–1147.
- Young, W.W.; I Hakomori, S.; Durdik, J.M.; Henney, C.S. Identification of ganglio-N-tetraosylceramide as a new cell surface marker for murine natural killer (NK) cells. J. Immunol. 1980, 124, 199–201.
- Habu, S.; Fukui, H.; Shimamura, K.; Kasai, M.; Nagai, Y.; Okumura, K.; Tamaoki, N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J. Immunol. 1981, 127, 34–38.
- Okuda, T. Dietary Control of Ganglioside Expression in Mammalian Tissues. Int. J. Mol. Sci. 2019, 21, 177.
- Okuda, T. A low-carbohydrate ketogenic diet promotes ganglioside synthesis via the transcriptional regulation of ganglioside metabolism-related genes. Sci. Rep. 2019, 9, 7627.
- Vos, J.P.; Lopes-Cardozo, M.; Gadella, B.M. Metabolic and functional aspects of sulfogalactolipids. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1994, 1211, 125–149.
- Okuda, T.; Numata, S.; Ito, M.; Ohta, M.; Kawamura, K.; Wiels, J.; Urano, T.; Tajima, O.; Furukawa, K.; Furukawa, K. Targeted Disruption of Gb3/CD77 Synthase Gene Resulted in the Complete Deletion of Globo-series Glycosphingolipids and Loss of Sensitivity to Verotoxins. J. Biol. Chem. 2006, 281, 10230–10235.
- Fujii, Y.; Numata, S.; Nakamura, Y.; Honda, T.; Furukawa, K.; Urano, T.; Wiels, J.; Uchikawa, M.; Ozaki, N.; Matsuo, S.; et al. Murine glycosyltransferases responsible for the expression of globo-series glycolipids: cDNA structures, mRNA expression, and distribution of their products. Glycobiology 2005, 15, 1257–1267.
- Magnani, J.L. The discovery, biology, and drug development of sialyl Lea and sialyl Lex. Arch. Biochem. Biophys. 2004, 426, 122–131.
- Nimrichter, L.; Burdick, M.M.; Aoki, K.; Laroy, W.; Fierro, M.A.; Hudson, S.A.; Von Seggern, C.E.; Cotter, R.J.; Bochner, B.S.; Tiemeyer, M.; et al. E-selectin receptors on human leukocytes. Blood 2008, 112, 3744–3752.
- St Hill, C.A. Interactions between endothelial selectins and cancer cells regulate metastasis. Front. Biosci. 2011, 16, 3233–3251.
- Kushi, Y.; Tsunoda, A.; Komatsuzaki, A.; Watanabe, K.; Kasama, T.; Handa, S. Characterization of Blood-Group-ABO(H)-Active Glycosphingolipids in Type-AB Human Erythrocytes. JBIC J. Biol. Inorg. Chem. 1995, 231, 862–867.
- Okuda, T.; Nakakita, S.; Nakayama, K. Structural characterization and dynamics of globotetraosylceramide in vascular endothelial cells under TNF-α stimulation. Glycoconj. J. 2010, 27, 287–296.
- Solter, D.; Knowles, B.B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc. Natl. Acad. Sci. USA 1978, 75, 5565–5569.
- Gooi, H.C.; Feizi, T.; Kapadia, A.; Knowles, B.B.; Solter, D.; Evans, M.J. Stage-specific embryonic antigen involves αl→ 3 fucosylated type 2 blood group chains. Nat. Cell Biol. 1981, 292, 156–158.
