Porcine reproductive and respiratory syndrome virus (PRRSV) affects the global swine industry and causes disastrous economic losses each year. The genome of PRRSV is an enveloped single-stranded positive-sense RNA of approximately 15 kb. The PRRSV replicates primarily in alveolar macrophages of pig lungs and lymphatic organs and causes reproductive problems in sows and respiratory symptoms in piglets. To date, studies on how PRRSV survives in the host, the host immune response against viral infections, and pathogenesis, have been reported. PRRSV vaccines have been developed, including inactive virus, modified live virus, attenuated live vaccine, DNA vaccine, and immune adjuvant vaccines. However, there are certain problems with the durability and effectiveness of the licensed vaccines. Moreover, the high variability and fast-evolving populations of this RNA virus challenge the design of PRRSV vaccines, and thus effective vaccines against PRRSV have not been developed successfully. As is well known, viruses interact with the host to escape the host’s immune response and then replicate and propagate in the host, which is the key to virus survival.
- PRRSV
- host
- interaction
- immune responses
- vaccines
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV) has undoubtedly become a substantial financial issue that has affected pig production and caused substantial economic losses for the swine industry worldwide since its outbreak in the 1980s [1,2]. Annual economic losses in the US due to PRRSV manifested in reproductive problems in sows and respiratory symptoms in piglets were assessed as reaching USD 664 million in 2013 [3,4]. The latest economic estimate in Germany indicated the losses on farm profits due to the PRRS virus were −19.1% on average and −41% in the worst case [2]. As a global swine pathogen that has caused catastrophic economic losses, PRRSV is the cause for continuing and widespread concern [5].
As a member of the Nidovirales order in the Arteriviridae family, PRRSV is an enveloped virus with an average diameter of 55 nm; it is a positive-stranded RNA virus with an approximately 15 kb genome with a 5′ cap and a 3′ poly A tail [4,6]. Except for the 5′ and 3′ untranslated regions at both ends, the PRRSV genome contains at least 11 known open reading frames (ORFs) [7]. The first two ORFs occupy about 75% of the viral genomes coding for polyproteins, including pp1a and pp1ab, by ribosome shifting, and then the PRRSV proteases hydrolyze and cleave polyproteins into at least 16 distinct nonstructural proteins (nsps) [4,8]. The nsps that participate in viral genome replication and transcription are essential for the survival of the PRRSV [9]. PRRSV virions are composed of an N protein (nucleocapsid protein) and a lipid envelop (GP2, E, GP3, GP4, M, GP5, ORF5a) that envelops the membrane [10,11]. M and GP5 are the major components of the virus coat [12].
The PRRSV is mainly divided into two genotypes: type 1 (represented by the European strain Lelystad Virus) and type 2 (represented by the North American strain VR-2332), and both PRRSV genotypes have only 50–60% nucleotide identity [6,13]. In addition to the genotype differences between PRRSV-1 and PRRSV-2, the host immune responses have been shown to differ, sometimes considerably due to their biological differences including pathogenicity (Table 1). A large number of studies have found a general consensus that PRRSV-2 causes more severe respiratory disease than PRRSV-1 [14]. Therefore, it is necessary to emphasize the host immune response induced by different genotypes. Due to high inter-strain genetic exchange and rapid mutations of PRRSV, it has always been a substantial challenge to design effective vaccines and drugs [4].
| Host | PRRSV | Function | Virus Genotypes | References |
|---|---|---|---|---|
| Heparan | M/GP5 | Concentrate virions on the cell surface | PRRSV-1/PRRSV-2 | [15] |
| pSn | M/GP5 | PRRSV attachment and internalization receptor via clathrin-mediated endocytosis | PRRSV-1 | [16] |
| CD163 (5JFB) | GP2/GP3/ GP4/GP5 |
Uncoating and genome release | PRRSV-1/PRRSV-2 | [13,17] |
| CD151 | 3′ UTR RNA | Cooperate in infection | PRRSV-2 | [18] |
| Simian vimentin | N (1P65) | Mediate transportation of the virus in the cytosol | PRRSV-2 | [19] |
| CD209 | GP5 | Essential in PRRSV entry and infection | PRRSV-1/PRRSV-2 | [20] |
| MYH9 | GP5 | Essential in PRRSV entry and infection | PRRSV-1/PRRSV-2 | [21,22] |
| IFN-β | nsp1α (3IFU) | Suppress IFN by degrading CBP | PRRSV-2 | [23,24] |
| nsp1β (3MTV) | Suppress IFN by inhibiting both IRF-3 and NF-κB-dependent gene induction | PRRSV-2 | [25] | |
| N | Suppress IFN by inhibiting the phosphorylation and nuclear translocation of IRF3 | PRRSV-2 | [26] | |
| nsp2 | Suppress IFN by inhibiting the activation of the IRF-3 and NF-κB signaling | PRRSV-2 | [27] | |
| nsp4 (5Y4L) | Suppress IFN by blocking NF-κB activation | PRRSV-2 | [28] | |
| nsp11 (5EYI) | Suppress IFN by inhibiting the activation of the IRF-3 and NF-κB signaling, and inhibiting the formation and nuclear translocation of ISGF3 targeting IRF9 |
PRRSV-2 | [29,30,31] | |
| IL-1β | E | Increase the release of IL-1β | PRRSV-2 | [32] |
| nsp11 | Inhibit the secretion of IL-1β | PRRSV-2 | [33] | |
| IL-10 | N | Up-regulate IL-10 via NF-κB and p38 MAPK pathways in PAMs | PRRSV-2 | [34] |
| nsp1 | Up-regulate IL-10 | PRRSV-2 | [35] | |
| Gp5 | Up-regulate IL-10 through p38 MAPK and signal transducer and activator of transcription-3 (STAT3) activation | PRRSV-2 | [36] | |
| IL-17 | nsp11 | Induced IL-17 production depending on the PI3K-p38MAPK-C/EBPβ/CREB pathways | PRRSV-2 | [37] |
| TRIM25 | N | Competitively interact with TRIM25, thereby interfering with TRIM25-mediated RIG-I ubiquitination | PRRSV-2 | [38] |
| TRIM22 | N | Interact with TRIM22 thereby reducing virus replication | PRRSV-2 | [39] |
| TRIM59 | nsp11 | Interact with TRIM59 thereby reducing virus replication | PRRSV-2 | [40] |
| MiR-181 | ORF4 | Inhibit PRRSV replication | PRRSV-2 | [41,42] |
| MiR-130 | 5′ UTR | PRRSV-2 | [43] | |
| MiR-23 | ORF3 | PRRSV-2 | [44] | |
| MiR-378 | ORF7 | PRRSV-2 | [44] | |
| MiR-505 | ORF3/ORF5 | PRRSV-2 | [44] | |
| PIAS1 SLA-I(5YLX) |
nsp1α | Modulate degradation via SUMO E3 ligase activity | PRRSV-2 PRRSV-2 |
[45] [45,46] |
| Nup62 | nsp1β | Inhibit host antiviral protein expression | PRRSV-1/PRRSV-2 | [47] |
| STAT3 | nsp5 | Promote the degradation of STAT3 and interference with the JAK/STAT3 signaling | PRRSV-1/PRRSV-2 | [48] |
| pRb | nsp9 | Benefit the replication of PRRSV | PRRSV-2 | [49] |
| NLRX1 | nsp9 | Restrict PRRSV replication | PRRSV-2 | [50] |
| ZAP | nsp9 | Repress PRRSV replication. | PRRSV-2 | [51] |
| Fibrillarin Nucleolin Poly(A)-binding |
N | Function remains to be further clarified | PRRSV-2 | [52] |
2. The Process of the PRRSV Entry and Infection
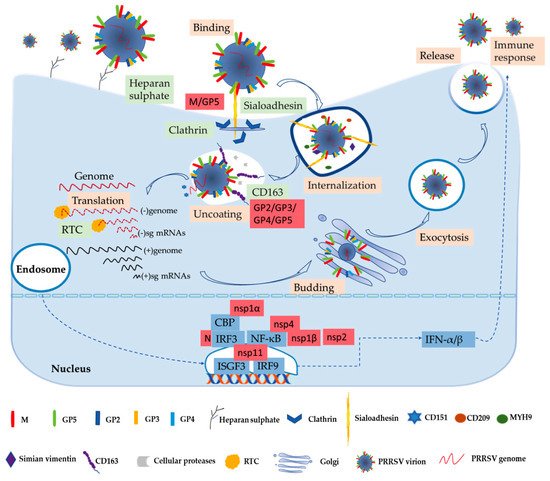
3. The PRRSV–Host Interactions
The complex networks of virus–host interactions are essential in the overall process of PRRSV entry and infection. The viral structural proteins interact with host receptors to mediate viral entry as described above. The interactions of host and viral nonstructural proteins can exert an influence on the replication and transcription of viral genomes. The virus’s invasion causes a series of immune responses, and then virions escape from the host immune system to favor their own replication by interacting with the host. Only by better understanding the molecular mechanism of PRRSV immune evasion and modulation can we design more effective vaccines. Further studies of these host–virus interactions are critical to the development of novel antiviral strategies against PRRSV.
3.1. Interferons (IFNs)
3.2. Interleukin (IL)
3.3. Tripartite Motif (TRIM) Proteins
3.4. MicroRNAs (miRNAs)
3.5. Other Host Factors’ Interactions with PRRSV
PRRSV depends on host factors to complete genome replication and interacts with host molecules for its survival and reproduction. The host immune system will take some measures to suppress the virus replication during virus invasion. Besides the abovementioned host factors, other cellular components are also involved in interacting with PRRSV. The cellular protein nucleoporin 62 (Nup62) interacts with nsp1β, leading to inhibition of host antiviral protein expression, revealing a new strategy of immune escape [47]. In addition, research found that cellular retinoblastoma protein (pRb) interacts with the nsp9 of genotype 2 PRRSV, which will benefit the replication of PRRSV-2 [49]. Inversely, the leucine-rich repeats (LRR) domain of nucleotide-binding oligomerization domain-like receptor (NLR) X1 as a new host restriction factor interacts with the RdRp domain of PRRSV-2 nsp9, which restricts PRRSV-2 replication [50]. Similarly, the interaction of the zinc finger domain of ZAP, a zinc finger antiviral protein, and nsp9 mapped to amino acids 150 to 160, can repress PRRSV-2 replication [51]. Nsp1α can combine with the protein inhibitor of activated STAT1 (PIAS1) as the small ubiquitin-related modifier (SUMO) E3 ligase leading to the nsp1α sumoylation [45]. In virtue of the SUMO E3 ligase activity, nsp1α interacts with swine leukocyte antigen class I (SLA-I) to modulate degradation, in the same way facilitating the ubiquitinylation of CBP for degradation [45,46]. The nsp5 of PRRSV-1 and PRRSV-2 was shown to promote the degradation of signal transducer and activator of transcription 3 (STAT3), a pleiotropic signaling mediator of numerous cytokines, leading to interference with the JAK/STAT3 signaling and the host immune responses [48]. The PRRSV-2 N protein interacts with RNA-associated nuclear host proteins such as fibrillarin, nucleolin, and poly(A)-binding protein, but the specific function remains to be further clarified [52]. Furthermore, the PRRSV N protein appears to up-regulate NF-κB activation that is attributed to the N protein nuclear localization signal [45].
In conclusion, a complex network of PRRSV–host interactions exists throughout the virus cycle, including virus entry, replication, and infection, as shown in Table 1. The immune response caused by different virus genotypes is emphasized in Table 1. Although significant advancements have been made, the understanding of direct or indirect virus–host interaction networks remain limited. Moreover, some seemingly contradictory results are hard to explain. Study indicated that the NF-κB is stimulated by the N protein, which may up-regulate IFN [45]. However, the N protein inhibits the phosphorylation and nuclear translocation of IRF3 to suppress IFNβ induction [26]. The stimulation of NF-κB by the N protein may involve other cellular pathways, and the mechanism of cytokines’ regulation by the N protein needs to be further explored. The interaction of PRRSV nsp9, a critical component of the viral RTC, and some host proteins can inhibit virus replications such as NLRX1 and ZAP, and yet they can promote virus replications such as pRb, which has aroused much concern.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines9040364
