Osteoarthritis (OA), a degenerative joint disorder, has been reported as the most common cause of disability worldwide. The production of inflammatory cytokines is the main factor in OA. Previous studies have been reported that obeticholic acid (OCA) and OCA derivatives inhibited the release of proinflammatory cytokines in acute liver failure, but they have not been studied in the progression of OA. In our study, we screened our small synthetic library of OCA derivatives and found T-2054 had anti-inflammatory properties.
- T-2054
- osteoarthritis
- RAW 264.7 cell line
- ATDC5 chondrocytes
- NF-κB-signaling
1. Introduction
Osteoarthritis (OA) is a degenerative joint disorder with main pathological features of progressive subchondral bone sclerosis, articular cartilage, osteophyte formation and synovial inflammation and has been reported as the most leading cause of disability worldwide. Osteoarthritis is more common in people over the age of 60. There is a grand challenge to treat OA [1,2,3,4,5].
OA’s progress is related to many factors, such as an imbalance of oxidant-antioxidant levels and the production of inflammatory cytokines. Production of inflammatory factors (TNF-α, IL-8 and IL-6) plays an important role in the pathogenesis, which caused arthritic pain and putative disease progression. Therefore, reducing the production of inflammatory cytokines has been regarded as a valid strategy for treating OA [1]. Until now, the treatment of OA remains a major challenge [5,6]. The inducible nitric oxide synthase (iNOS) has been demonstrated to be one of the key inflammatory mediators, participates in the release of nitric oxide (NO) and binds to cyclooxygenase-2 (COX-2) to regulate inflammatory response [7,8]. Articular cartilage injury is the main factor leading to developing OA. The pathological features of articular cartilage injury include chondrocyte apoptosis and a decrease in the ability to synthesize extracellular matrix (ECM), which mediated catabolic cytokines by increasing the expression of matrix metalloproteinases (MMPs) and ADAMTS [9,10,11,12].
Nuclear factor kappa-B (NF-κB) signaling pathway, a transcription factor, is directly activated, which results in the production of cytokines (TNF-α, IL-8 and IL-6) and NO in OA [13]. Moreover, the NF-κB-signaling pathway plays an important role in articular cartilage damage by regulating hypoxia-inducible factor-2α (HIF-2α) transcriptional activity [14]. The phosphorylation of NF-κB p65 is the key element in the activation of the NF-κB-signaling pathway. Many studies report that blocking the NF-κB-signaling pathway could attenuate developing OA [13].
In recent years, a large number of new pathology [4,15]. It has been reported that low approaches have been explored to treat OA. For example, anti-inflammatory drugs, Traditional Chinese medicine (TCM) or mesenchymal stem cell therapies were applied to relieve pain, and slow progression doses of methotrexate (MTX) may benefit OA through various mechanisms [16]. However, the adverse effects, cost and potential risks of these therapies need to be further investigated.
Obeticholic acid (OCA (6-ethyl-chenodeoxycholic acid)), a semisynthetic bile acid analog, binds with a high affinity for the farnesoid X receptor (FXR) to suppress lipogenesis and reduce de novo cholesterol biosynthesis [17]. Moreover, OCA relieved insulin resistance in an obese model and protected rats from gut barrier dysfunction in a cholestasis model [18,19]. It is reported that OCA and OCA derivatives inhibited the expression of hepatic proinflammatory cytokines in GalN/LPS-induced acute liver failure [20]. However, the relationship between OCA derivatives and OA is still not clear.
2. Inhibitory Activity on LPS-Induced NO Release
Structures and synthesis of OCA and OCA derivatives were showed (Figure 1a, Supplementary Materials). Nitric oxide (NO), an inflammatory mediator, is highly produced in response to inflammatory stimuli [21]. The release of NO was tested to investigate the anti-inflammatory effect in LPS-induced RAW 264.7 cells. Among the tested compounds, T-2054 observably inhibited LPS-induced NO release (IC50 value: 0.484 ± 0.127 μM) in RAW 264.7 cells compared with the LPS group (Figure 1b,c).
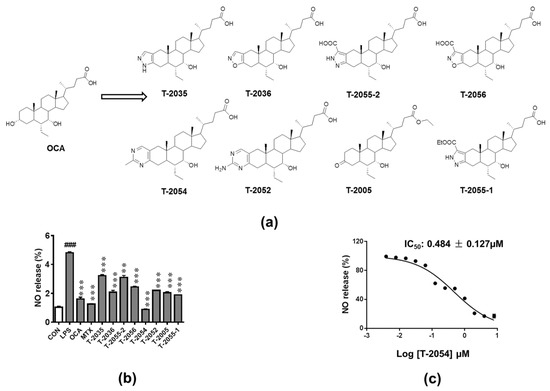
Figure 1. Detect the release of nitric oxide (NO). Structures of obeticholic acid (OCA) and OCA derivatives (a). The RAW-264.7 cells were pretreated with compounds and methotrexate (MTX) (0.5 μM) for 3 h before lipopolysaccharides (LPS) stimulation. 24 h later, the amounts of NO in the culture supernatants were measured by Griess agents (b). The NO inhibition rates curve of T-2054, with IC50 values of 0.484 ± 0.127 μM (c). Each value represents the mean ± SD, performed in 3 different experiments. ### p < 0.001 vs. normal control (CON); *** p < 0.001 vs. LPS group.
3. Effect of T-2054 on Cell Survival in RAW 264.7 Cells and ATDC5 Cells
Before exploring the therapeutic effects of T-2054, the cell viability was tested by the CCK-8 kit to explore the potential cytotoxicity on RAW 264.7 cells and ATDC5 cells. Our data showed no obvious cytotoxicity was observed after T-2054 treatment at different concentrations (0, 0.1, 1, 10, 100 μM) in both RAW 264.7 cells and ATDC5 cells. Thus, the optimal concentration was chosen in subsequent experiment processes (Figure 2a–c).
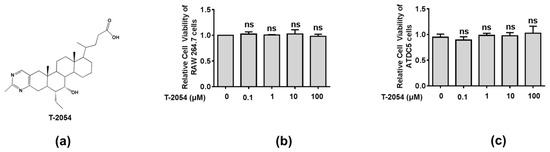
Figure 2. Detect the cell viability of RAW 264.7 cell and ATDC5 cell. Chemical structure of T-2054 (a). The RAW 264.7 cell and ATDC5 cell viability were measured by CCK-8 kits after exposure to different concentrations of T-2054 for 24 h (b,c). Each value represents the mean ± SD, performed in 3 different experiments. ns vs. T-2054 (0 μM).
4. T-2054 Inhibited the Expression of IL-6, IL-8 and TNF-α in LPS-Induced RAW 264.7 Cells
The production of inflammatory cytokines is the main cause of OA and leads to the activation of various signaling pathways to aggravate the symptoms [22,23,24]. The inhibitory effects of T-2054 on gene and protein levels of IL-6, IL-8 and TNF-α were determined by real-time quantitative PCR and enzyme-linked immunosorbent assay (ELISA). It was shown that the release of inflammatory cytokines was obviously elevated by lipopolysaccharides (LPS). On the contrary, T-2054 suppressed the expression of these inflammatory cytokines. These results showed that T-2054 certainly attenuated inflammatory response in LPS-activated RAW 264.7 cells (Figure 3a–f).
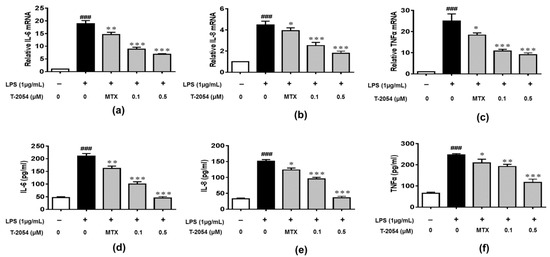
Figure 3. Gene and protein expression of IL-6, IL-8 and TNF-α. The RAW 264.7 cells were treated with LPS (1 μg/mL) 1 h later, T-2054 was added for 6 h. The total RNA was collected, and mRNA levels of IL-6 (a), IL-8 (b) and TNF-α (c) were analyzed by qPCR. The RAW 264.7 cells were exposed to LPS (1 μg/mL) for 1 h before T-2054 was added. After 16 h, supernatants were collected, and levels of IL-6 (d), IL-8 (e) and TNF-α (f) were analyzed by ELISA. Actin was used as an internal control to normalize the data. Each value represents the mean ± SD, performed in 3 different experiments. ### p < 0.001 vs. CON group; * p < 0.05; ** p < 0.01; *** p < 0.001 vs. LPS group.
5. Discussion
At present, osteoarthritis remains a chronic disease affecting daily activities, particularly among old people, which was mediated by inflammation. Production of inflammatory factors, osteoporosis, trauma, obesity and joint malformation are the main factors in developing OA [30,31]. Acetaminophen and nonsteroidal anti-inflammatory drugs are commonly used clinical drugs to treat OA. However, there have been few effective therapies to withdraw the progress of OA [32]. Recently, a number of anti-inflammatory drugs were applied to the patient. For example, rographolide, licorice, and kaempferol have been reported to possess anti-inflammatory properties [33,34,35,36]. Though these agents could relieve pain and retard the progress of OA, they possess adverse effects, such as gastrointestinal, hepatic, and cardio-renal damage [37]. Hence, anti-inflammatory drugs with effective clinical effects and no deleterious effects have raised interest in the treatment of OA [28,29].
Farnesoid X receptor (FXR) plays an important regulatory role in various inflammatory diseases, such as chronic heart failure, inflammatory bowel disease, and hepatic inflammation [38,39,40]. Obeticholic acid (OCA), a farnesoid X receptor (FXR) agonist, prevented mice from LPS-induced liver injury, ameliorated liver injury induced by carbon tetrachloride via inhibiting inflammatory activity and alleviated the symptoms of acute liver injury [4,17,20].
In the present study, we reported that T-2054, a derivative of OCA, had an anti-osteoarthritis property by screening our small synthetic library of OCA derivatives. Subsequently, we evaluated the effect of T-2054 on the production of proinflammatory cytokines/chemokines (IL-6, IL-8 and TNF-α) and the change of chondrocyte features. IL-6, a proinflammatory cytokine, could lead to the degradation of cartilage and bone; IL-8 is a chemokine recruiting immune cells and leukocyte to pathological tissue; TNF-α, released by activated macrophages, is regarded as a vital proinflammatory cytokine in OA [41,42]. These proinflammatory cytokines further exaggerate chondrocyte apoptosis and decrease the ability to synthesize extracellular matrix (ECM) [10]. Moreover, the production of NO, due to the increased production/activity of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), results in cartilage tissue damage [43]. In this study, we found that T-2054 obviously reduced the amount of NO in the culture media, the release of proinflammatory cytokines (IL-6, IL-8 and TNF-α), and the expression of iNOS and COX-2 in LPS-induced RAW 264.7 cells. LPS treatment is a common method to simulate an inflammatory reaction in macrophages. In addition, LPS treatment is regarded as an ideal model to test the anti-inflammatory effects of several compounds [44]. Moreover, T-2054 could relieve cartilage degradation and destruction via increasing SOX9 expression and decreasing MMP9 and ADMTS5 expression, which are tightly related to cartilage degradation and destruction in TNF-α-induced ATDC5 cells [14].
In our in vivo studies, T-2054 reduced cytokines’ expression (IL-6, IL-8 and TNF-α) in serum and cartilage degradation and destruction in the OA mice model. The therapeutic effect was far better than that of MTX (2 mg/kg). Moreover, there was no significant difference in the effect on body weight and organ damage after treatment with T-2054, suggesting that T-2054 would be safe and effective for the clinical treatment of OA.
It is reported that transcription factors NF-κB play an important role in the expression of proinflammatory cytokines and cartilage damage, which are the main factors for developing OA [14,45]. In our research, we found the phosphorylation level of P65 was decreased after treated with T-2054 in LPS-activated RAW 264.7 cells and TNF-α-induced ATDC5 cells. These data suggested that T-2054 may act as an inhibitor of NF-κB to suppress inflammatory response and cartilage damage in OA.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22083807
