SLC6A14 and SLC38A5 are the two transporters that are upregulated in a variety of cancers to mediate the influx of glutamine, serine, glycine, and methionine into cancer cells. SLC6A14 is a Na+/Cl− -coupled transporter for multiple amino acids, including these four amino acids. In contrast, SLC38A5 is a Na+-coupled transporter with rather restricted specificity towards glutamine, serine, glycine, and methionine. Both transporters exhibit unique functional features that are ideal for the rapid proliferation of cancer cells.
- cancer-specific metabolism
- one-carbon metabolism
- glutamine addiction
- oncometabolites
- amino acid transporters
- SLC6A14 and SLC38A5
1. SLC6A14 and SLC38A5 and Their Relevance to Cancer
Cancer cells exhibit increased demands for all amino acids, essential as well as non-essential. Since the essential amino acids cannot be generated de novo, these have to be obtained solely from extracellular sources. In contrast, the non-essential amino acids can be produced endogenously in cells from other precursors; nonetheless, cancer cells rely on extracellular sources to a significant extent even for these amino acids because their synthetic capacity does not meet the highly increased demands. Therefore, cancer cells upregulate specific amino acid transporters in their plasma membrane to mediate the entry of essential and non-essential amino acids from blood. To date, four distinct amino acid transporters have received increasing attention for their role in the promotion of cancer: SLC1A5 (also known as ASCT2), SLC7A5 (also known as LAT1), SLC7A11 (also known as xc−), and SLC6A14 (also known as ATB0,+) [1][2][3][4][5]. SLC38A5 (also known as SN2) [6][7] should also be added to this elite list prompted by the findings that it is a target for the c-Myc oncogene [8], but relatively much less is known on this transporter in cancer compared to the other four transporters. Among these five amino acid transporters that have received attention with regard to their biological relevance to cancer, SLC6A14 and SLC38A5 stand out in terms of their functional features that are ideal for the promotion of cancer (Table 1).
Table 1. Functional features of SLC6A14 and SLC38A5 that distinguish these two transporters from other amino acid transporters known to be upregulated in cancer.
| Feature | SLC6A14 | SLC38A5 | SLC7A5 | SLC1A5 | SLC7A11 |
|---|---|---|---|---|---|
| Transport of Glutamine | + | + | + | + | - |
| Transport of all essential AA | + | − | + | − | − |
| Transport of mTOR activator Leu | + | − | + | − | − |
| Transport of Ser and Gly | + | + | − | + | − |
| Transport of Methionine | + | + | + | − | − |
| Transport of Cystine | − | − | − | − | + |
| Energy from membrane potential | + | + | − | − | − |
| Uniport of AA into cells | + | + | − | − | − |
| Mitogenic alkalinization | − | + | − | − | − |
| Macropinocytosis | − | + | − | − | − |
SLC6A14 and SLC38A5 mediate the transfer of their amino acid substrates in one direction, mostly into the cells in the influx mode (Figure 1). SLC6A14 is also known as ATB0,+, referring to its identity with the amino acid transport system B0,+ (B stands for “broad-specific”; uppercase letter stands for Na+-dependence; 0,+ in the superscript stands for the ability of the transporter to accept neutral and cationic amino acids as substrates). It is energized by three driving forces: a Na+ gradient, a Cl− gradient, and membrane potential [9][10][11]. Therefore, this transporter primarily mediates the influx of its substrates into cells because of the high magnitude of the combined driving forces. SLC38A5 is also known as SN2 (i.e., the second isoform of the amino acid transport system N that is capable of accepting the amino acids glutamine, asparagine, and histidine, which contain nitrogen in the side chain; N stands for nitrogen in the side chain) and SNAT5 (sodium-coupled neutral amino acid transporter 5). It is also driven by a Na+ gradient, but interestingly it is an electroneutral transporter with no involvement of membrane potential. This is because of the coupling of the transport process with a transmembrane H+ gradient in which the transfer of Na+ and the amino acid substrate in one direction is functionally coupled to the transfer of H+ in the opposite direction [6][7][12]. As SLC38A5 accepts only neutral amino acids as its substrates, the transport process is electroneutral. Based on this operational mechanism, the transfer of amino acids and Na+ into cells via SLC38A5 leads to the removal of H+ from the cells, thus resulting in intracellular alkalinization [7]. Stated differently, SLC38A5 functions as an amino acid-dependent Na+/H+ exchanger. Since the transmembrane Na+ gradient is quantitatively the principal driving force for SLC38A5 under normal physiologic conditions, this transporter could also mediate the efflux of glutamine from the cells if the cells express high levels of glutamine synthetase, thus causing a concentration gradient for the amino acid across the plasma membrane in the outward direction. This is the case for the function of this transporter in astrocytes in the brain and Muller cells in the retina where SLC38A5 mediates the release of glutamine from the cells with subsequent uptake of glutamine by neurons; this is a part of the glutamine–glutamate cycle that operates between astrocytes/Muller cells and neurons [13][14]. However, the functioning of this transporter in the glutamine efflux mode is unique to astrocytes/Muller cells. This transporter is expected to function solely in the influx mode in cancer cells because of the avid utilization of glutamine by these cells, thus creating a concentration gradient for the amino acid across the plasma membrane that facilitates influx rather than efflux. In contrast to SLC6A14 and SLC38A5, the other three transporters related to cancer are all amino acid exchangers, meaning that the influx of one amino acid substrate into cells is coupled to the efflux of another amino acid substrate out of the cells [3]. Consequently, these three transporters are not ideal to meet the increased demands for amino acids in cancer cells because when the cells acquire one given amino acid via these transporters, they lose some other amino acid.
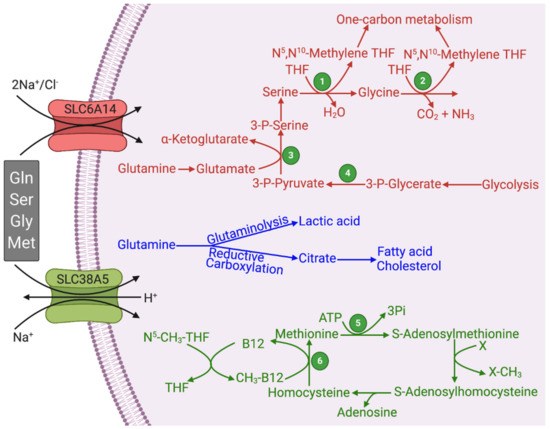
Figure 1. Functional features of SLC6A14 and SLC38A5 and their relevance to glutaminolysis and serine–glycine–one-carbon pathway. Figure legend 1, Serine hydroxymethyl transferase (SHMT); 2, Glycine cleavage system; 3, Phosphoserine aminotransferase (PSAT); 4, Phosphoglycerate dehydrogenase (PHGDH); 5, Methionine synthase; THF, Tetrahydrofolate.
The substrate selectivity of SLC6A14 and SLC38A5 bears a key connection to glutaminolysis and serine–glycine–one-carbon pathway. Both transporters transport glutamine, serine, glycine, and methionine (Figure 1; Table 1). Some functional features do distinguish the two transporters. SLC6A14 transports 18 of the 20 proteinogenic amino acids, including all essential amino acids; as leucine is a potent activator of oncogenic mTOR signaling, the functional activity of this transporter is coupled to mTOR activation. Both of these features are essential for cancer cells to support their growth and proliferation. In contrast, the substrate selectivity of SLC38A5 is relatively restricted. This transporter can mediate the uptake of only glutamine, asparagine, histidine, serine, glycine, and methionine; this list of substrates contains all the four amino acids related to glutaminolysis and one-carbon metabolism. However, SLC38A5 exhibits a functional feature that is not seen with SLC6A14. The transport function of SLC38A5 is coupled to H+ efflux. As cancer cells generate massive amounts of lactic acid, there is an absolute need for these cells to prevent intracellular acidification, and SLC38A5 contributes to this process. The resultant intracellular alkalinization promotes the entry of the cells into the DNA-synthesis phase of the cell cycle and consequently accelerates cell proliferation [15][16]. It has been documented that SLC38A5 causes an increase in pH in the immediate vicinity of the plasma membrane on the cytoplasmic side of the cell in the presence of its amino acid substrates [7]. This effect is expected to promote another nutrient delivery system, known as macropinocytosis, in cancer cells. The NHE1 isoform (SLC9A1) of the Na+/H+ exchanger is known for its mitogenic potential with its role in the prevention of intracellular acidification as well as in the promotion of macropinocytosis [17]; inhibitors of NHE1 are widely used to block macropinocytosis [18]. The alkaline pH on the cytoplasmic surface of the plasma membrane influences the phosphorylation and activity of cytoskeletal scaffold proteins to initiate and promote macropinocytosis, a non-selective mode of uptake of nutrients from the extracellular milieu by “drinking” minute droplets of extracellular fluid [19][20][21][22]. As mentioned earlier, SLC38A5 functions as an amino acid-dependent Na+/H+ exchanger. Therefore, this transporter is expected to promote macropinocytosis in the presence of its amino acid substrates, such as glutamine, serine, glycine, asparagine, histidine, and methionine. It has already been documented that increased activity of macropinocytosis is a hallmark of certain types of solid tumors, particularly tumors associated with KRAS mutations [21][22].
2. Potential Functional Coupling between SLC6A14/SLC38A5 with Other Transporters
Since glutamine is a good substrate for SLC6A14 and SLC38A5, the Na+-coupled concentrative influx of these amino acids into cancer cells is likely to activate the heterodimeric amino acid transporter known as cystine/glutamate exchanger (SLC7A11/4F2hc). This exchanger mediates the influx of the disulfide amino acid cystine into cells coupled to the efflux of the anionic amino acid glutamate. Glutamine concentrated inside the cells via SLC6A14 and SLC38A5 is expected to generate glutamate either by glutaminases in the glutaminase I pathway or by glutamine-dependent transaminases in the glutaminase II pathway. This glutamate would provide the exchange amino acid to facilitate the influx of cystine via SLC7A11/4F2hc. This would represent an important biological process for the cancer cells because cystine entering the cells will be converted into cysteine within the cells, which along with glutamate and glycine, will form glutathione, thus enhancing the antioxidant machinery in cancer cells. In a similar manner, it is also possible that SLC38A5 and the H+-coupled lactate transporter MCT1 (SLC16A1) are functionally coupled in cancer cells via H+. The transport function of SLC38A5 involves the influx of Na+ and amino acid substrates into cells coupled to the efflux of H+. It is known that lactate generated in hypoxic cancer cells that are located far away from blood supply is used as an energy substrate by oxygenated cancer cells that lie in close proximity to blood vessels; this process involves the entry of lactate via MCT1 into lactate-using cells coupled to H+ influx. This H+ would serve as a substrate for SLC38A5 to increase the transporter’s ability to mediate the influx of amino acids into the cells. The vice versa is also possible in which the H+ efflux mediated by SLC38A5 fuels the entry of lactate/H+ via MCT1. Such a mutual stimulation of the two transporters would be an ideal phenomenon to support cancer cell survival and proliferation. Nonetheless, the aforementioned ideas on the functional coupling between SLC6A14/SLC38A5 and SLC7A11/4F2hc and between SLC38A5 and MCT1 remain only speculative at this time and have to wait for experimental supporting evidence.
3. Upregulation of SLC6A14 and SLC38A5 in Cancer Cells and Signaling Mechanisms Involved in the Process
Several reports have shown marked upregulation of SLC6A14 in many types of solid tumors, including colon cancer [23][24], cervical cancer [25][26], estrogen receptor-positive breast cancer [27][28][29], and pancreatic cancer [30][31][32][33][34][35]. The role of SLC6A14 as a potential biomarker for survival and prognosis has been validated, particularly in pancreatic ductal adenocarcinoma, with higher expression levels in tumors correlating with reduced survival [30][31][32][33][34][35]. These findings strongly suggest that SLC6A14 functions as a tumor promoter. The signaling mechanisms responsible for the upregulation of this transporter in cancer are just beginning to be understood. Estrogen induces the expression of the transporter [28], thus providing a molecular mechanism for the upregulation of the transporter, specifically in estrogen receptor-positive breast cancer. The involvement of Wnt signaling in the upregulation of SLC6A14 has been described recently [24]. Signaling pathways initiated by Wnt ligands are known to be associated with carcinogenesis [36][37]. Therefore, it seems that the upregulation of SLC6A14 with its tumor-promoting capability represents at least one of the molecular mechanisms by which estrogen signaling and Wnt signaling drive carcinogenesis and tumor growth.
Relatively much less is known on the expression and function of SLC38A5 in cancer. A recent report has indicated a significant correlation between the expression level of this transporter in cancers and resistance to cisplatin efficacy [38]. The Cancer Genome Atlas (TCGA) database provides evidence for the upregulation of SLC38A5 at least in pancreatic cancer and an inverse correlation between the expression levels of the transporter and survival in patients with this cancer. Despite the paucity of data on SLC38A5 in cancer, there is convincing evidence for the role of this transporter in promoting cell proliferation. In the intestinal tract, this transporter is expressed specifically in crypt cells where it provides the cells with glutamine and other amino acids [39]; these cells normally exhibit a high proliferative capacity. The expression of the transporter is induced under pathological conditions associated with increased expansion of the intestinal epithelial cells [40][41][42]. Single-cell transcriptomic profiling has shown SLC38A5 as a characteristic gene for the progenitors of glucagon-secreting α-cells [43]. Amino acid delivery via this transporter drives α-cell hyperplasia [44][45], and the elevation of amino acids in blood induces a subpopulation of α-cells to form pancreatic neuroendocrine tumors [46]. Interestingly, similar to SLC6A14, the expression of SLC38A5 is also under the control of the Wnt signaling pathway [47][48][49], thus suggesting a probable connection between this transporter and cancer.
4. SLC6A14 and SLC38A5 as Actionable Drug Targets for Cancer Therapy
SLC6A14 and SLC38A5 are cell-surface proteins that support amino acid nutrition in cancer cells. The amino acids supplied by these transporters serve not only as the building blocks for protein synthesis but also function as signaling molecules (e.g., mTOR activation) and provide substrates for the cancer-cell-specific metabolic pathways, such as glutaminolysis and serine–glycine–one-carbon pathway. As such, SLC6A14 and SLC38A5 drive cancer cell proliferation and tumor growth in specific types of solid tumors. In theory, if small molecules can be developed that have high affinity and high selectivity to inhibit the transport function of these transporters, such inhibitors could potentially have efficacy as anticancer drugs. This idea has already been validated with α-methyl-L-tryptophan as a fairly selective inhibitor of SLC6A14 [27]. It is important to note that α-methyl-L-tryptophan is not a transportable substrate for SLC6A14; it binds to the substrate-binding site of the transporter and blocks the transport function without itself being transported into the cells. As such, α-methyl-L-tryptophan is a blocker of the transporter rather than a competitive inhibitor. The IC50 value for the L-enantiomer to block the transport function of SLC6A14 is ~10 μM. With this small molecule, published reports have demonstrated the therapeutic utility of SLC6A14 blockade in the treatment of multiple tumor types that are associated with upregulation of this transporter: breast cancer [27][28][29], pancreatic cancer [31][50], and colon cancer [24].
There have been some studies describing selective inhibitors of SLC38A5 [51]; the best inhibitor in terms of selectivity is glutamate-γ-hydroxamate. It is a competitive inhibitor of the transporter, but it is not known whether the compound is actually transported into cells via the transporter. Furthermore, the inhibitor shows low affinity with an IC50 value of ~0.6 mM. Even though selective high-affinity inhibitors/blockers for SLC38A5 are not yet available, the experience with α-methyl-L-tryptophan and SLC6A14 indicates that a similar approach would also work for SLC38A5-positive tumors as well. Another feasible approach is to generate monoclonals specific to extracellular epitopes of these transporters that could be used to block the function of these cell-surface proteins. Even though it is widely acknowledged that amino acid metabolism comprising of numerous biochemical pathways is obligatory for cancer cell survival and that amino acid transporters play an essential role in supplying the amino acid substrates to feed into these pathways, the possibility of exploiting these transporter proteins as actionable drug targets for cancer therapy is just beginning to be recognized. If successful, this would represent a novel, hitherto unexplored, therapeutic approach for the treatment of cancer.
This entry is adapted from the peer-reviewed paper 10.3390/ph14030216
References
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009, 121, 29–40.
- Bhutia, Y.D.; Ganapathy, V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim. Biophys. Acta Bioenergy 2016, 1863, 2531–2539.
- Bhutia, Y.D.; Babu, E.; Ramachandran, S.; Ganapathy, V. Amino Acid Transporters in Cancer and Their Relevance to “Glutamine Addiction”: Novel Targets for the Design of a New Class of Anticancer Drugs. Cancer Res. 2015, 75, 1782–1788.
- Cormerais, Y.; Vučetić, M.; Parks, S.K.; Pouyssegur, J. Amino Acid Transporters Are a Vital Focal Point in the Control of mTORC1 Signaling and Cancer. Int. J. Mol. Sci. 2020, 22, 23.
- Scalise, M.; Pochini, L.; Galluccio, M.; Console, L.; Indiveri, C. Glutamine transporters as pharmacological targets: From function to drug design. Asian J. Pharm. Sci. 2020, 15, 207–219.
- Nakanishi, T.; Sugawara, M.; Huang, W.; Martindale, R.G.; Leibach, F.H.; Ganapathy, M.E.; Prasad, P.D.; Ganapathy, V. Structure, Function, and Tissue Expression Pattern of Human SN2, a Subtype of the Amino Acid Transport System N. Biochem. Biophys. Res. Commun. 2001, 281, 1343–1348.
- Nakanishi, T.; Kekuda, R.; Fei, Y.-J.; Hatanaka, T.; Sugawara, M.; Martindale, R.G.; Leibach, F.H.; Prasad, P.D.; Ganapathy, V. Cloning and functional characterization of a new subtype of the amino acid transport system N. Am. J. Physiol. Physiol. 2001, 281, C1757–C1768.
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.-Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787.
- Ganapathy, M.E.; Ganapathy, V. Amino Acid Transporter ATB0,+ as a Delivery System for Drugs and Prodrugs. Curr. Drug Targets Immune Endocr. Metab. Disord. 2005, 5, 357–364.
- Sikder, M.O.F.; Yang, S.; Ganapathy, V.; Bhutia, Y.D. The Na+/Cl−-Coupled, Broad-Specific, Amino Acid Transporter SLC6A14 (ATB0,+): Emerging Roles in Multiple Diseases and Therapeutic Potential for Treatment and Diagnosis. AAPS J. 2017, 20, 12.
- Nałęcz, K.A. Amino Acid Transporter SLC6A14 (ATB0,+)—A Target in Combined Anti-cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 594464.
- Bröer, S. The SLC38 family of sodium–amino acid co-transporters. Pflügers Arch. Eur. J. Physiol. 2013, 466, 155–172.
- Umapathy, N.S.; Li, W.; Mysona, B.A.; Smith, S.B.; Ganapathy, V. Expression and function of glutamine transporters SN1 (SNAT3) and SN2 (SNAT5) in retinal Muller cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3980–3987.
- Jenstad, M.; Chaudhry, F.A. The Amino Acid Transporters of the Glutamate/GABA-Glutamine Cycle and Their Impact on Insulin and Glucagon Secretion. Front. Endocrinol. 2013, 4, 199.
- Oberleithner, H.; Schwab, A.; Westphale, H.-J.; Wojnowski, L. Oscillations: A key event in transformed renal epithelial cells. J. Mol. Med. 1992, 70, 816–824.
- Flinck, M.; Kramer, S.H.; Pedersen, S.F. Roles of pH in control of cell proliferation. Acta Physiol. 2018, 223, e13068.
- Pedersen, S.F. The Na+/H+ exchanger NHE1 in stress-induced signal transduction: Implications for cell proliferation and cell death. Pflügers Arch. Eur. J. Physiol. 2006, 452, 249–259.
- Devadas, D.; Koithan, T.; Diestel, R.; Prank, U.; Sodeik, B.; Döhner, K. Herpes Simplex Virus Internalization into Epithelial Cells Requires Na+/H+ Exchangers and p21-Activated Kinases but neither Clathrin- nor Caveolin-Mediated Endocytosis. J. Virol. 2014, 88, 13378–13395.
- Palm, W. Metabolic functions of macropinocytosis. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180285.
- Stow, J.L.; Hung, Y.; Wall, A.A. Macropinocytosis: Insights from immunology and cancer. Curr. Opin. Cell Biol. 2020, 65, 131–140.
- Derle, A.; de Santis, M.C.; Gozzelino, L.; Ratto, E.; Martini, M. The role of metabolic adaptation to nutrient stress in pancreatic cancer. Cell Stress 2018, 2, 332–339.
- Pupo, E.; Avanzato, D.; Middonti, E.; Bussolino, F.; Lanzetti, L. KRAS-Driven Metabolic Rewiring Reveals Novel Actionable Targets in Cancer. Front. Oncol. 2019, 9, 848.
- Gupta, N.; Miyauchi, S.; Martindale, R.G.; Herdman, A.V.; Podolsky, R.; Miyake, K.; Mager, S.; Prasad, P.D.; Ganapathy, M.E.; Ganapathy, V. Upregulation of the amino acid transporter ATB0,+ (SLC6A14) in colorectal cancer and metastasis in humans. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1741, 215–223.
- Sikder, M.O.F.; Sivaprakasam, S.; Brown, T.P.; Thangaraju, M.; Bhutia, Y.D.; Ganapathy, V. SLC6A14, a Na+/Cl−-coupled amino acid transporter, functions as a tumor promoter in colon and is a target for Wnt signaling. Biochem. J. 2020, 477, 1409–1425.
- Gupta, N.; Prasad, P.D.; Ghamande, S.; Moore-Martin, P.; Herdman, A.V.; Martindale, R.G.; Podolsky, R.; Mager, S.; Ganapathy, M.E.; Ganapathy, V. Up-reguation of the amino acid transporter ATB0,+ (SLC6A14) in carcinoma of the cervix. Gynecol. Oncol. 2006, 100, 8–13.
- Royse, K.E.; Zhi, D.; Conner, M.G.; Clodfelder-Miller, B.; Srinivasasainagendra, V.; Vaughan, L.K.; Skibola, C.F.; Crossman, D.K.; Levy, S.; Shrestha, S. Differential gene expression landscape of co-existing cervical pre-cancer lesions using RNA-seq. Front. Oncol. 2014, 4, 339.
- Karunakaran, S.; Umapathy, N.S.; Thangaraju, M.; Hatanaka, T.; Itagaki, S.; Munn, D.H.; Prasad, P.D.; Ganapathy, V. Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem. J. 2008, 414, 343–355.
- Karunakaran, S.; Ramachandran, S.; Coothankandaswamy, V.; Elangovan, S.; Babu, E.; Periyasamy-Thandavan, S.; Gurav, A.; Gnanaprakasam, J.P.; Singh, N.; Schoenlein, P.V.; et al. SLC6A14 (ATB0,+) Protein, a Highly Concentrative and Broad Specific Amino Acid Transporter, Is a Novel and Effective Drug Target for Treatment of Estrogen Receptor-positive Breast Cancer. J. Biol. Chem. 2011, 286, 31830–31838.
- Babu, E.; Bhutia, Y.D.; Ramachandran, S.; Gnanaprakasam, J.P.; Prasad, P.D.; Thangaraju, M.; Ganapathy, V. Deletion of the amino acid transporter Slc6a14 suppresses tumour growth in spontaneous mouse models of breast cancer. Biochem. J. 2015, 469, 17–23.
- Penheiter, A.R.; Erdogan, S.; Murphy, S.J.; Hart, S.N.; Felipe-Lima, J.; Rakhshan-Rohakhtar, F.; O’Brien, D.R.; Bamlet, W.R.; Wuertz, R.E.; Smyrk, T.C.; et al. Transcriptomic and immunohistochemical profiling of SLC6A14 in pancreatic ductal adenocarcinoma. BioMed Res. Int. 2015, 2015, 593572.
- Coothankandaswamy, V.; Cao, S.; Xu, Y.; Prasad, P.D.; Singh, P.K.; Reynolds, C.P.; Yang, S.; Ogura, J.; Ganapathy, V.; Bhutia, Y.D. Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br. J. Pharmacol. 2016, 173, 3292–3306.
- Cheng, Y.; Wang, K.; Geng, L.; Sun, J.; Xu, W.; Liu, D.; Gong, S.; Zhu, Y. Identification of candidate diagnostic and prognostic biomarkers for pancreatic carcinoma. EBioMedicine 2019, 40, 382–393.
- Li, Y.; Zhu, Y.; Dai, G.; Wu, D.; Gao, Z.; Zhang, L.; Fan, Y. Screening and validating the core biomarkers in patients with pancreatic ductal adenocarcinoma. Math. Biosci. Eng. 2020, 17, 910–927.
- Roche, S.; O’Neill, F.; Murphy, J.; Swan, N.; Meiller, J.; Conlon, N.T.; Geoghegan, J.; Conlon, K.; McDermott, R.; Rahman, R.; et al. Establishment and characterization by expression microarray of patient-derived xenograft panel of human pancreatic adenocarcinoma patients. Int. J. Mol. Sci. 2020, 21, 962.
- Chang, X.; Yang, M.-F.; Fan, W.; Wang, L.-S.; Yao, J.; Li, Z.-S.; Li, D.-F. Bioinformatic Analysis Suggests That Three Hub Genes May Be a Vital Prognostic Biomarker in Pancreatic Ductal Adenocarcinoma. J. Comput. Biol. 2020, 27, 1595–1609.
- Sharma, M.; Pruitt, K. Wnt Pathway: An Integral Hub for Developmental and Oncogenic Signaling Networks. Int. J. Mol. Sci. 2020, 21, 8018.
- Zhong, Z.; Yu, J.; Virshup, D.M.; Madan, B. Wnts and the hallmarks of cancer. Cancer Metastasis Rev. 2020, 39, 625–645.
- Girardi, E.; César-Razquin, A.; Lindinger, S.; Papakostas, K.; Konecka, J.; Hemmerich, J.; Kickinger, S.; Kartnig, F.; Gürtl, B.; Klavins, K.; et al. A widespread role for SLC transmembrane transporters in resistance to cytotoxic drugs. Nat. Chem. Biol. 2020, 16, 469–478.
- Talukder, J.R.; Kekuda, R.; Saha, P.; Prasad, P.D.; Ganapathy, V.; Sundaram, U. Functional characterization, localization, and molecular identification of rabbit intestinal N-amino acid transporter. Am. J. Physiol. Liver Physiol. 2008, 294, G1301–G1310.
- Saha, P.; Arthur, S.; Kekuda, R.; Sundaram, U. Na-glutamine co-transporters B0AT1 in villus and SN2 in crypts are differentially altered in chronically inflamed rabbit intestine. Biochim. Biophys. Acta Biomembr. 2012, 1818, 434–442.
- Singh, S.; Arthur, S.; Talukder, J.; Palaniappan, B.; Coon, S.; Sundaram, U. Mast cell regulation of Na-glutamine co-transporters B0AT1 in villus and SN2 in crypt cells during chronic intestinal inflammation. BMC Gastroenterol. 2015, 15, 1–8.
- Singh, S.; Arthur, S.; Sundaram, U. Unique regulation of Na-glutamine cotransporter SN2/SNAT5 in rabbit intestinal crypt cells during chronic enteritis. J. Cell. Mol. Med. 2017, 22, 1443–1451.
- Stanescu, D.E.; Yu, R.; Won, K.-J.; Stoffers, D.A. Single cell transcriptomic profiling of mouse pancreatic progenitors. Physiol. Genom. 2017, 49, 105–114.
- Kim, J.; Okamoto, H.; Huang, Z.; Anguiano, G.; Chen, S.; Liu, Q.; Cavino, K.; Xin, Y.; Na, E.; Hamid, R.; et al. Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic α Cell Hyperplasia in Mice. Cell Metab. 2017, 25, 1348–1361.e8.
- Dean, E.D.; Li, M.; Prasad, N.; Wisniewski, S.N.; von Deylen, A.; Spaeth, J.; Maddison, L.; Botros, A.; Sedgeman, L.R.; Bozadjieva, N.; et al. Interrupted Glucagon Signaling Reveals Hepatic α Cell Axis and Role for L-Glutamine in α Cell Proliferation. Cell Metab. 2017, 25, 1362–1373.e5.
- Smith, D.K.; Kates, L.; Durinck, S.; Patel, N.; Stawiski, E.W.; Kljavin, N.; Foreman, O.; Sipos, B.; Solloway, M.J.; Allan, B.B.; et al. Elevated Serum Amino Acids Induce a Subpopulation of Alpha Cells to Initiate Pancreatic Neuroendocrine Tumor Formation. Cell Rep. Med. 2020, 1, 100058.
- Schafer, N.F.; Luhmann, U.F.O.; Feil, S.; Berger, W. Differential gene expression in Ndph-knockout mice in retinal develop-ment. Investig. Ophthalmol. Vis. Sci. 2009, 50, 906–916.
- Xia, C.-H.; Yablonka-Reuveni, Z.; Gong, X. LRP5 Is Required for Vascular Development in Deeper Layers of the Retina. PLoS ONE 2010, 5, e11676.
- Chen, J.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Joyal, J.-S.; Juan, A.M.; Hatton, C.J.; Aderman, C.M.; Dennison, R.J.; Willett, K.L.; et al. Retinal Expression of Wnt-Pathway Mediated Genes in Low-Density Lipoprotein Receptor-Related Protein 5 (Lrp5) Knockout Mice. PLoS ONE 2012, 7, e30203.
- Cai, A.; Zheng, H.; Chen, Z.; Lin, X.; Li, C.; Yao, Q.; Bhutia, Y.D.; Ganapathy, V.; Chen, R.; Kou, L. Synergism between SLC6A14 blockade and gemcitabine in pancreactic cancer: A 1H-NMR-based metabolomic study in pancreatic cancer cells. Biochem. J. 2020, 477, 1923–1937.
- Low, S.Y.; Taylor, P.M.; Ahmed, A.; Pogson, C.I.; Rennie, M.J. Substrate-specificity of glutamine transporters in membrane vesicles from rat liver and skeletal muscle investigated using amino acid analogues. Biochem. J. 1991, 278, 105–111.
