Potato germplasm is characterized by a huge variability in composition and concentration of secondary metabolites that play a role in increasing plant ability to cope with environmental challenges, due to their reported biocide activity on insects, bacteria, and fungi. Their distribution within the tuber is not uniform: Most of them are concentrated in the peel, made of periderm tissue, whose cell layers contain corky cell walls, which confer protection from phytopathogens, especially during tuber growth and storage. Thus, considering that potato peel is constantly exposed to biotic stresses, it is not surprising that it is a precious source of bioactive compounds, mainly phenolics and alkaloids, which have an enormous potential to deliver new bioprotectors.
- sustainable food industry
- waste residues
- potato peel
- antifungal activity
- mycotoxigenic fungi control
- cereal protection
- circular economy
1. Potato Glycoalkaloids
Steroidal glycoalkaloids (SGAs), a class of nitrogen-containing steroid glyosides, are the most important alkaloids in the Solanaceae family [1]. In potato germoplasm, there are more than 80 different molecules [2][3][4], but in cultivated potato, α-chaconine (β-d-Glucopyranoside, (3β)-solanid-5-en-3-yl O-6-deoxy-α-l-mannopyranosyl-(1-2)-O- (6-deoxy-α-l-mannopyranosyl-(1-4)), and α-solanine (solanid-5-en-3β-yl α-l-rhamnopyranosyl -(1→2)-[β-d-glucopyranosyl- (1→3)]-β-d-galactopyranoside) account for up to 95% of the total tuber SGAs [5][6]. SGA structures share a common aglycone, a six-ring steroid skeleton (solanidine), to which a branched triose is attached. The triose is a sugar moiety attached to the 3-position of the first ring and a nitrogen atom in the sixth ring end of the molecule: In the case of α-chaconine it consists of β-chacotriose (bis-α-l-rhamnopyranosyl-β-d-glucopyranose), while for α-solanine, it is a β-solatriose (α-l-rhamnopyranosyl-β-d-glucopyranosyl-β-galactopyranose (Figure 1B) [5]. The SGA biosinthetic pathway has not yet been completely determined, even though it putatively derives from isopropanoid pathway. Cholesterol has been identified as metabolic precursor: It is cyclized into solanidine, a steroidal alkaloid (SA) that is subsequently glycosylated to α-solanine and α-chaconine [7]. A similar SGA, tomatine, has been found in tomato and eggplants, with small but significant structural differences. It has a branched chain composed of lycotetraose, a tetrasaccharide constituted by two glucose units, xylose and galactose (22S,25S)-5α-spirosolan-3β-yl-β-d-glucopyranosyl-(1→2)-[β-d-xylopyranosyl-(1→3)]-β-d-glucopyranosyl-(1→4)-β-d-galactopyranoside [5].
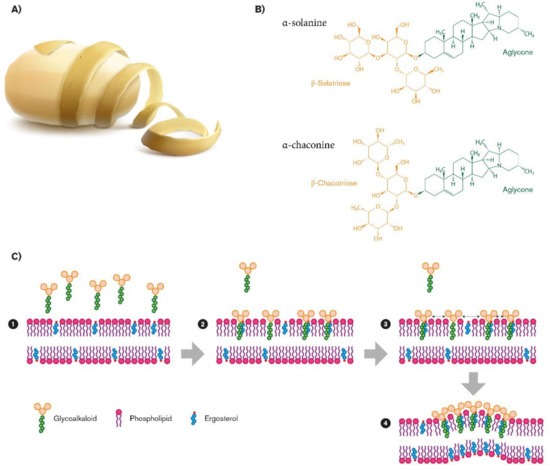
Figure 1. Biovalorization of glycoalkaloids α-chaconine and α-solanine from potato peel waste (A). Their chemical structures (B), and their role in fungal membrane disruption (C).
The distribution of SGAs in plant is not uniform, including leaves, tubers, roots, and sprouts, especially in green parts, flowers, and fruits [5]; their accumulation is affected by many different factors, including developmental and environmental conditions such as high temperature [8], light exposure [9], light quality [10], and wounding [11], but it also depends on genotype [12]. SGA content in the peel was often reported to be high, ranging between 0.83 and 352.6 mg/100 gr DW (dry weight) in eight potato cultivars [13] and resulting in 50 mg/100 gr DW in cv Bionica [14]. Two interesting studies indicated that this variability could be correlated with flesh color: White-flesh potatoes showed higher content of SGAs in peel than blue-flesh, red-flesh, and yellow-flesh potatoes [15]. White-flesh potatoes can reach up to 3526 mg Kg−1, blue-flesh potatoes 245 mg Kg−1, red-flesh potatoes 1264 mg Kg−1, and yellow-flesh potatoes 425 mg Kg−1 FW (fresh weight) [16].
SGAs are considered toxic to humans: Daily oral doses from 3 to 6 mg GAs/kg body weight can even be lethal [17]. The widely accepted safety limit in tubers is 20 mg/100 g FW, even though a maximum level has not been officially established at the EU level [12]. A recent opinion from the German Federal Institute for Risk Assessment (BfR) suggests lowering the safety limit in potatoes to less than 10 mg/100 g FW [18]. However, most edible mature tubers contain low amounts of SGAs in their flesh [19], as a consequence of their selective reduction by breeding due to potato domestication for human consumption. Commonly commercialized potatoes rarely exceed the recommended SGAs level, with some exceptions such as cv. Lenape and cv. Bonum that were consequently withdrawn from the US and Swedish markets [20][21].
2. Potato Phenols
Thousands of phenolic compounds have been isolated in the plant kingdom with a role of either oxidative stress protectors or as pest control agents, and have been classified in several subgroups based on their structure: Phenolic acids, flavonoids, tannins, coumarins, lignans, quinones, stilbens, and curcuminoids. Their structure contains hydroxylated aromatic rings, with the hydroxy group being attached directly to the phenyl, substituted phenyl, or different aryl group. Phenolic compounds are synthesized via the shikimic acid and phenylpropanoid pathways. Potato peel is a well-established source of phenolic acids and flavonoids: Their content varies, respectively, among 1.02–2.92 g/100 g and 0.51–0.96 g/100 g DW [22] and significantly decreases toward the skin close sections (cortex) and flesh, as confirmed either by direct measurement, such as HPLC analysis [14], and indirect measurement, such as the radical scavenging activity [23]. The most abundant phenolic compound is chlorogenic acid, CGA (5-O-caffeoylquinic acid; 5-CQA), together with its isomers (3,4-diCQA, 3,5-diCQA, and 4,5-diCQA) [5]. CGA was also truly called 3-caffeoylquinic acid before 1976, when, according to new IUPAC rules, the CA structure changed in 5-caffeoylquinic acid and the 3-caffeoylquinic acid isomer was referred to as neochlorogenic acid [24]. CGA resulted in nearly 2115 µg g−1 DW in skin, decreasing in adjacent cortex to 276 µg g−1 DW (cv. Bionica) [14]. Conversely, hydroxycinnamic and hydroxybenzoic acids are present only in trace, with the exception of caffeic acid ((2E)-3-(3,4-dihydroxyphenyl) prop-2-enoic acid; CA). CA and its derivatives, and catechin (C6-C3-C6), belonging, respectively, to the class of hydroxycinnamics (C6-C3) and flavonoids, specifically flavan-3-ols, resulting in the lowest concentration in the tuber.
CA possesses relevant antioxidant activity both in vitro and in vivo, higher than that observed for CGA [25] and it can be accumulated in plants, mainly in conjugated forms due to esterification by quinic acid (1S,3R,4S,5R)-1,3,4,5-tetrahydroxycyclohexane -1-carboxylic acid), in two configurational isomers, such as trans or cis. In biological systems, the most relevant form is the trans isomer, because of its best stability at subacid pH in the plant microenvironment [26], even though its photoexcitation for the absorption in the UVA region (400-315 nm) can lead from trans to cis structure [27]. Its ortho-diphenolic moiety confers important antioxidant features to CA: Lowering the OH bond dissociation enthalpy, thereby increasing the rate of H-atom transfer to peroxyl [28]. Moreover, the ortho-diphenolic system has a relatively higher oxidative reaction rate, with the negative secondary effect of the tuber turning brown when cut or damaged, with the essential contribution of molecular O2 and of specific enzymatic activities. The molecular mechanism of the browning phenotype is not yet completely elucidated [29].
Catechin (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol) is an ortho-diphenolic belonging to the flavan-3-ol family. Differently from other phenol compounds, catechin is found in free form. Peculiarly, it can be condensed in oligomers or in higher molecular weight polymers, commonly named “condensed tannic compounds”, present in black tea and red wine [30]. Its chemical structure confers interesting antioxidant properties, higher than those of other common biological antioxidants, such as glutathione and ascorbate [31]. Although its level in peel is lower compared to CA, catechin might be investigated as a potential bioprotector due to its antimicrobial and antifungal activities [32].
This entry is adapted from the peer-reviewed paper 10.3390/molecules26082174
References
- Friedman, M.; McDonald, G.M.; Filadelfi-Keszi, M. Potato Glycoalkaloids: Chemistry, Analysis, Safety, and Plant Physiology. CRC Crit. Rev. Plant. Sci. 1997, 16, 55–132.
- Kozukue, N.; Yoon, K.S.; Byun, G.I.; Misoo, S.; Levin, C.E.; Friedman, M. Distribution of glycoalkaloids in potato tubers of 59 accessions of two wild and five cultivated Solanum species. J. Agric. Food Chem. 2008, 56, 1920–1928.
- Manrique-Carpintero, N.C.; Tokuhisa, J.G.; Ginzberg, I.; Holliday, J.A.; Veilleux, R.E. Sequence diversity in coding regions of candidate genes in the glycoalkaloid biosynthetic pathway of wild potato species. G3 Genes Genomes Genet. 2013, 3, 1467–1479.
- Mweetwa, A.M.; Hunter, D.; Poe, R.; Harich, K.C.; Ginzberg, I.; Veilleux, R.E.; Tokuhisa, J.G. Steroidal glycoalkaloids in Solanum chacoense. Phytochemistry 2012, 75, 32–40.
- Friedman, M. Potato Glycoalkaloids and Metabolites: Roles in the Plant and in the Diet. J. Agric. Food Chem. 2006, 54, 8655–8681.
- Distl, M.; Wink, M. Identification and Quantification of Steroidal Alkaloids from Wild Tuber-Bearing Solanum Species by HPLC and LC-ESI-MS. Potato Res. 2009, 52, 79–104.
- Itkin, M.; Heinig, U.; Tzfadia, O.; Bhide, A.J.; Shinde, B.; Cardenas, P.D.; Bocozeba, S.E.; Unger, T.; Malitsky, S.; Finkers, R.; et al. Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science 2013, 341, 175–179.
- Lafta, A.M.; Lorenzen, J.H. Influence of High Temperature and Reduced Irradiance on Glycoalkaloid Levels in Potato Leaves. J. Am. Soc. Hort. Sci. 2000, 25, 563–566.
- Dale, M.F.B.; Griffiths, D.W.; Bain, H.; Todd, D. Glycoalkaloid increase in Solanum tuberosum on exposure to light. Ann. Appl. Biol. 1993, 123, 411–418.
- Mekapogu, M.; Sohn, H.-B.; Kim, S.-J.; Lee, Y.-Y.; Park, H.-M.; Jin, Y.-I.; Hong, S.-Y.; Suh, J.-T.; Kweon, K.; Jeong, J.-C.; et al. Effect of Light Quality on the Expression of Glycoalkaloid Biosynthetic Genes Contributing to Steroidal Glycoalkaloid Accumulation in Potato. Am. J. Potato Res. 2016, 93, 264–277.
- Choi, D.; Bostock, R.M.; Avdiushko, S.; Hildebrand, O.F. Lipid-derived signals that discriminate wound- and pathogenresponsive isoprenoid pathways in plants: Methyl jasmonate and fungal elicitor arachidonic acid induce different 3-hydroxy3-methylglutaryl-coenzyme A reductase genes and antimicrobial isoprenoids in Solanum tuberosum L. Proc. Natl. Acad. Sci. USA 1994, 91, 2329–2333.
- Zarins, R.; Kruma, Z. Glycoalkaloids in potatoes: A review. Foodbalt 2017, 7–11.
- Friedman, M.; Roitman, J.N.; Kozukue, N. Glycoalkaloid and calystegine contents of eight potato cultivars. J. Agric. Food Chem. 2003, 51, 2964–2973.
- Pacifico, D.; Musmeci, S.; Sanchez del Pulgar, J.; Onofri, C.; Parisi, B.; Sasso, R.; Mandolino, G.; Lombardi-Boccia, G. Caffeic acid and α-chaconine influence the resistance of potato tuber to Phthorimaea operculella (Lepidoptera: Gelechiidae). Am. Potato J. 2019, 96, 403–413.
- Friedman, M. Analysis of biologically active compounds in potatoes (Solanum tuberosum L.), tomatoes (Lycopersicon esculentum L.), and jimson weed (Datura stramonium L.) seeds. J. Chromatogr. A 2005, 1054, 143–155.
- Rytel, E.; Czopek, A.T.; Aniolowska, M.; Hamouz, K. The influence of dehydrated potatoes processing on the glycoalkaloids content in coloured-fleshed potato. Food Chem. 2013, 141, 2495–2500.
- OECD. Consensus document on compositional considerations for new varieties of potatoes: Key food and feed nutrients, anti-nutrients and toxicants. In OECD Environmental Health and Safety Publications, Safety of Novel Foods and Feeds No. 4; OECD: Paris, France.
- BfR Opinion No 010/2018 of 23 April 2018. Table Potatoes Should Contain Low Levels of Glycoalkaloids (Solanine). Available online: (accessed on 20 February 2021).
- Uluwaduge, D.I. Glycoalkaloids, bitter tasting toxicants in potatoes: A review. Int. J. Food Sci. Nutr. 2018, 3, 188–193.
- Hellenäs, K.E.; Branzell, C.; Johnsson, H.; Slanina, P. High levels of glycoalkaloids in the established Swedish potato variety Magnum Bonum. J. Sci. Food Agric. 1995, 68, 249–255.
- Knuthsen, P.; Jensen, U.; Schmidt, B.; Larsen, I.K. Glycoalkaloids in potatoes: Content of glycoalkaloids in potatoes for consumption. J. Food Compos. Anal. 2009, 22, 577–581.
- Calcio Gaudino, E.; Colletti, A.; Grillo, G.; Tabasso, S.; Cravotto, G. Emerging Processing Technologies for the Recovery of Valuable Bioactive Compounds from Potato Peels. Foods 2020, 9, 1598.
- Wu, Z.G.; Xu, H.Y.; Ma, Q.; Cao, Y.; Ma, J.N.; Ma, C.M. Isolation, identification and quantification of unsaturated fatty acids, amides, phenolic compounds and glycoalkaloids from potato peel. Food Chem. 2012, 135, 2425–2429.
- Kremr, D.; Bajer, T.; Bajerová, P.; Surmová, S.; Ventura, K. Unremitting problems with chlorogenic acid Nomenclature: A review. Quím. Nova 2016, 39, 530–533.
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138.
- Clifford, M.N. Chlorogenic acids and other cinnamates—nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043.
- Horbury, D.; Baker, L.A.; Quan, W.D.; Greenough, S.E.; Stavros, W.G. Photodynamics of potent antioxidants: Ferulic and caffeic acids. Phys. Chem. Chem. Phys. 2016, 18, 17691–17697.
- Lucarini, M.; Pedulli, G.F. Bond dissociation enthalpy of α-tocopherol and other phenolic antioxidants. J. Org. Chem. 1994, 59, 5063–5070.
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Quantification Methods and Mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248.
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117.
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem 2018, 241, 480–492.
- Veluri, R.; Weir, T.L.; Bais, H.P.; Stermitz, F.R.; Vivanco, J.M. Phytotoxic and Antimicrobial Activities of Catechin Derivatives. J. Agric. Food Chem. 2004, 52, 1077–1082.
