In biomedical applications, scaffolds can be used ranging from regenerative engineering to controlled drug delivery and immunomodulation, and for this purpose, biomaterials have become an indispensable instrument as scaffold material. The materials used for scaffold manufacturing must satisfy some criteria such as intrinsic biofunctionality and appropriate chemistry to stimulate molecular biorecognition by cells to induce proliferation, cell adhesion, and activation. Many biodegradable polymers of natural and synthetic origin have been established for use as biomaterials and careful consideration of the cellular environment and interactions needed is required to select a polymer for a given application.
- scaffolds
- tissue engineering
- natural biopolymer
- synthetic biopolymer
- biodegradability
1. Introduction
Tissue engineering (TE) is the in vitro construction of bioartificial tissues and in vivo modification of cell growth and function through the implantation of appropriate cells isolated from donor tissues to generate biocompatible scaffold materials [1]. This approach specifically focuses on the vital imbalance between the rising number of patients waiting for organ transplantation due to end-stage failure and a limited number of donated organs available for those procedures [2]. TE and regenerative medicine integrate information and technology from various fields such as genetics, engineering, pharmaceutics, medicine, chemistry, and materials sciences to perform treatments or to restore or replace damaged tissues and organs [3][4][5]. It holds the promise of sustainable development due to ever-going improvement in biomaterials and implies the procedure of fusing scaffolds, molecules, and cells that are biologically active into functional tissues. The ultimate goal is to completely monitor, create a functional structure/support to repair, preserve, or improve damaged tissues or entire organs and to implement “enhanced and sustainable quality of life (QOL) with health” as stated in the prime goal of the World Health Organization (WHO) [6][7].
In this field, two primary approaches are used to generate engineered tissues. Primarily, scaffolding is used as a cell supporting system for seeding cells in vitro, and further cells are stimulated to set up the matrix for building a tissue base for transplantation. The latter entails the use of a scaffold as a drug delivery device or a growth factor. This approach combines scaffolding with growth factors, and the body implant cells are recruited around the matrices at the scaffold sites to form the neo-tissue. Both methods do not preclude one another and can be easily fused [8][9][10][11].
Owing to its remarkable merits, TE is often believed to be the ultimate ideal medical treatment. This process is multi-stage and requires the development of various components to create the desired neo-tissues or organs. In several current strategies, biomaterials are essential components. The recent development of TE involves the preparation of new biomaterials that can meet the local environment and indications. Advanced technologies are now available to fabricate biomaterials (natural/synthetic) in designing scaffolds which support the formation of complex 3D tissues, many of them with functional vascular networks that match their in vivo counterparts [12][13].
Designing and manufacturing of the scaffold are important areas of biomaterial research for TE and regenerative medicine. [14]. Much work has been done over the past two decades to improve potentially relevant scaffold materials for TE. For neo-tissue generation in vitro and during the initial phase and after implantation, these scaffolds provide mechanical support and encourage cell growth and differentiation [15][16][17]. To date, many materials have predominantly been used to create biodegradable scaffolds comprising polymers with the synthetic origin [18] such as poly(α-hydroxy esters) including poly(ƹ-caprolactone) (PCL), polyglycolic acid (PGA), polylactic acid (PLA), and their copolymer poly(glycolic acid) (PLGA); poly(ethers) containing poly(ethylene oxide) (PEO) and poly(ethylene glycol) (PEG), polyvinyl alcohol (PVA), polyurethane (PU), etc. In addition, naturally occurring biomaterials like polypeptides and polysaccharides are also studied [19]. Composites or blending of these synthetic or natural polymers or together can provide a variety of physicochemical and biological characteristics [20]. Scaffold materials are defined in terms of mechanical characteristics, chemical composition, and degradation mechanisms. Biomaterial selection plays an important part in the design and production of medical implants and TE products [21]. Although the classical selection criteria for a healthy, durable implant is known as the choice of passive inert material, any artificial material placed in a patient’s body also generates a cellular response [22]. Therefore, it is now recognized that instead of behaving simply as an inert body, a biomaterial must be biologically suitable and interact with the tissue when implanted [23][24].
2. Scaffolding and Its Importance in Biomedical Applications (Regenerative Engineering)
The term “scaffold” refers to an artificial temporary platform applied to support, repair, or to enhance the performance of a structure. This can be done on different size and length scales, with various methods of support depending on the form and use. In general, two-dimensional studies of biomaterial substrates are carried out to test cell–biomaterial interactions. However, to ensure the functions of the damaged tissues, the scaffold is needed to replace the defect or mimic the organs or tissue structures in a three-dimensional manner [25]. Biocompatibility, biodegradability, mechanical characteristics, pore size, porosity, osteoinductivity, osteoconductivity, osteogenesis, and osteointegration are the key design considerations for the scaffold [26][27]. Some of the essentials of scaffolds used in TE are illustrated in Figure 1. After implemented in a body, the scaffold should aim to (i) be a liable structure for adhesion, proliferation, and cell differentiation as a substratum, (ii) create the required biomechanical environment for coordinated regeneration of tissues, (iii) permit the dissemination of nutrients and oxygen, and (iv) allow cells to be encapsulated and released with growth factors [28].
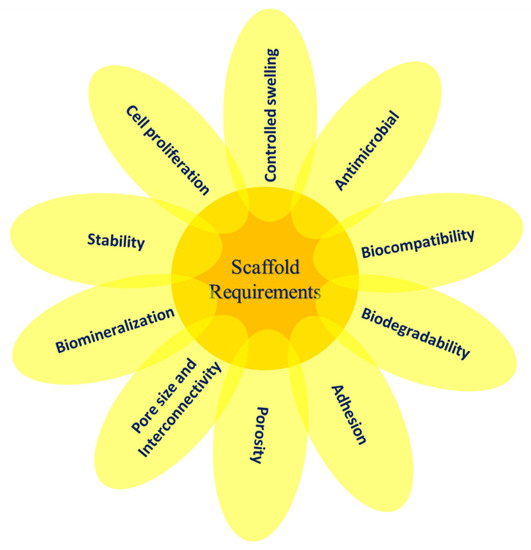
Figure 1. The essential variables involved in scaffold design for TE.
In TE along with regenerative medicine, scaffolds may act as delivery vectors or as cellular systems for drugs and cells. The other choice is to combine scaffolds with different cell types that can enhance osteogenic lineage tissue formation in vivo or release unique soluble lineage molecules. Before being implanted into the target site, these cells can be expanded selectively ex vivo. Scaffolds in clinical medicine are upcoming areas of considerable significance. They are typically associated with organ disease or failure conditions and used to repair organs to restore normal functionality [29][30]. It is well-known that scaffolds support and promote growth of regenerative cells and perform a major role in TE efficiency. Besides, the scaffolding biomaterial facilitates proliferation, differentiation, cell adhesion, offers mass transport and temporary 3D mechanical support, and finally causes the formation of neo-tissue (newly formed tissue built around a scaffold) [31].
In TE applications, the biological crosstalk between the scaffold and the cells is controlled by the properties of the materials and final scaffold characteristics. Materials used for scaffold manufacturing must have intrinsic biofunctionality and appropriate chemistry to stimulate molecular biorecognition from cells to induce proliferation, cell adhesion, and activation. The mechanical properties of the scaffold and kinetics of decomposition in selected materials must be adjusted to the TE application, specifically to ensure the essential structural features and to achieve the rate of new tissue formation. The final effectiveness of the regenerative process plays a major role in scaffolding, exposed surface area, pore distribution, and porosity, the quantity and distribution of which affect the rate of cell penetration within the scaffold volume and the architecture of the extracellular matrix (ECM) formed [23][32][33][34].
Scaffold design for tissue engineering includes several specifications. Many of these parameters are dynamic and not yet well-comprehended. Besides, these scaffolds should possess sufficient mechanical properties to provide neo-tissues with the necessary stress environment. To enable the entrance of nutrients into cells, the scaffolds should be porous, permeable, and have to demonstrate the required surface structure and chemistry for cell attachment [35]. These scaffolds can be created with natural or synthetic polymers or with bio-based ceramics or any suitable combinations.
3. Polymers as Biomaterials for Scaffolding
Any substance or a blend of the natural or synthetic source may be used in total or as part of any tissue, organ, or body function to maintain or to enhance, at any time, the person’s quality of life, and then that substitute can be assessed as a biomaterial [36].
In biomedical applications, scaffolds can be used ranging from regenerative engineering to managed drug delivery and immunomodulation; biomaterials have become an indispensable instrument [37]. Regenerative engineering is a multidisciplinary research area that uses the concepts of physics, stem cell science, advanced materials science, clinical translation, and developmental biology for damaged tissue regeneration [38][39].
While several biodegradable polymers are established for use as biomaterials, careful consideration of the particular cellular environment and interactions desired is essential in selecting a polymer for a given application. Applications of this type may include [40]:
-
Support for new tissue growth.
-
Prevention of cellular activity.
-
Guided tissue response.
-
Improvement of cell connection and consequent cellular activation.
-
Inhibition of cellular attachment and/or activation.
-
Prevention of a biological response.
Depending on the intended application, scaffold materials can be natural or synthetic, degradable or nondegradable. The polymer’s properties depend on their constituent macromolecules’ structure, composition, and arrangement. The principal forms of polymers used as biomaterials are biologically natural polymers, synthetic biodegradable and nonbiodegradable polymers as shown in Figure 2. Because of their specific characteristics, such as a wide range of biodegradation rates, high porosity with various pore sizes, high surface-to-volume ratio, and mechanical property, polymeric scaffolds attract great interest. They offer distinct benefits of biofunctionality, flexibility, and biological properties that are essential in TE and biomedical applications [31][41][42][43].
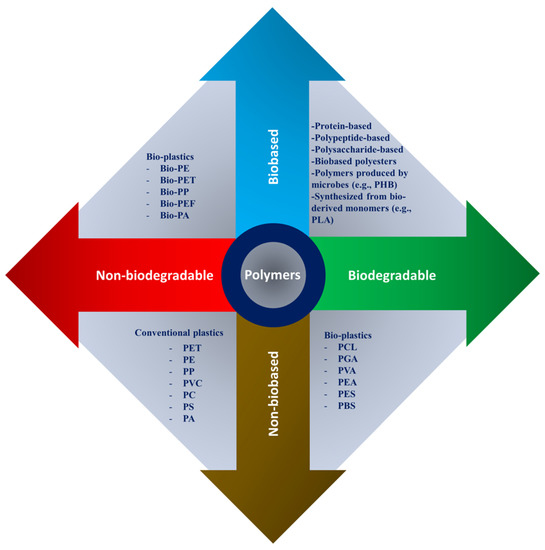
Figure 2. Natural and synthetic polymers were rearranged based on bio vs non-bio and biodegradable vs nonbiodegradable characteristics, where PHB: polyhydroxybutyrate; PLA: polylactic acid; PCL: polycaprolactone; PGA: poly(glycolic acid); PVA: poly(vinyl alcohol); PEA: poly(ethylene adipate); PES: polyethersulfone; PBS: polybutylene succinate; PET: polyethylene terephthalate; PE: polyethylene; PP: polypropylene; PVC: polyvinyl chloride; PC: polycarbonate; PS: polystyrene; PA: polyamide; and PEF: polyethylene furanoate.
3.1. Natural Biopolymer-Based Scaffolds
Natural biopolymers have resurged over the past few decades as primary bioactive substances used in the applications of medical materials. Based on their monomeric units and structure, biopolymers are categorized roughly into three classes [27][44]:
-
Polypeptide- and protein-based: collagen, fibrin, fibrinogen, gelatin, silk, elastin, myosin, keratin, and actin.
-
Polysaccharide-based: chitin, chitosan, alginate, hyaluronic acid, cellulose, agarose, dextran, and glycosaminoglycans.
-
Polynucleotide-based: DNA, linear plasmid DNA, and RNA.
These consist of long chains, including nucleotides, amino acids, or monosaccharides made of repeating covalently bonded groups. Biofunctional molecules which ensure bioactivity, biomimetic nature, and natural restructuring are typically found in such polymers. Bioactivity, biocompatibility, 3D geometry, antigenicity, non-toxic byproducts of biodegradation, and intrinsic structural resemblance are the most important properties of natural polymers [38]. Conversely, their key disadvantages, microbial contamination (i.e., endotoxins), decreased tunability, immunogenic reaction, uncontrollable rate of degradation, and poor mechanical strength restrict their application for hard tissue regeneration. Natural polymers make important contributions to TE, especially in the manufacture of scaffolds for therapeutic agent delivery. Novel and natural polymeric materials are aimed at enhancing different therapies due to their inherent bioactivity, biocompatibility, and bioresorbability [31][45]. Naturally derived polymers including collagen, chitin, chitosan, gelatin, silk fibroin, soybean, fibrinogen (Fbg), fibrin (Fbn), elastin, proteoglycan, hyaluronan, and laminin have displayed great potential in the biomedical section.
3.2. Synthetic Biopolymer-Based Scaffolds
Synthetic polymers are advantageous in a few characteristics such as tunable properties, endless forms, and established structures over natural polymers. The support offered by synthetic biomaterials can enable restoration of damaged or diseased tissue structure and function. Polymerization, interlinkage, and functionality (changed by block structures, by combining them, by copolymerization) of their molecular weight, molecular structure, physical and chemical features make them easily synthesized as compared to naturally occurring polymers [46][47]. The disadvantages of synthetic biomaterials are that they lack cell adhesion sites and require chemical modifications to enhance cell adhesion. Many commercially available synthetic polymers exhibit similar physicochemical and mechanical characteristics to biological tissues. In biodegradable polymers, synthetic polymers are a major category and can be produced under controlled conditions. In a broad spectrum, the mechanical and physical characteristics are predictable and reproducible, such as strength, Young’s modulus, and degradation rate. Poly(α-hydroxy esters) including PCL, PGA, PLA, and their copolymer PLGA and poly(ethers) including PEO and PEG, PVA, and PU are the most widely studied degradable synthetic materials. These are probably the most popular examples, although there are currently many other synthetic materials being sought [48][49][50]. These polymers have various levels of biodegradability, biocompatibility, and mechanical properties, but no single polymer holds all three of these critical properties at the optimum level [51].
A variety of natural and synthetic bio-polymeric substances used for biomedical scaffolding applications particularly towards regenerative engineering are tabulated in Table 1 along with their advantages and disadvantages.
Table 1. Natural and synthetic biopolymers along with their advantages and disadvantages.
|
Polymer |
Structure |
Desirable Properties and |
Disadvantages |
Ref |
||
|
Natural polymer-, |
Polypeptide-, and Protein-based scaffolds |
Collagen |
Triple helical structure held together by hydrogen bonds. Major amino acid groups include: |
Favorable for cell adhesion, proliferation, differentiation, and ECM secretion. Excellent biocompatibility. Biodegradability. Low toxicity. Rough surface morphology. Low immunogenicity. Weak antigenicity. |
Low mechanical strength. Difficult disinfection. The deformation and contraction of collagen-based scaffolds have restricted their use in load-bearing tissues. Poor stability in an aqueous environment. Potential for antigenicity through telopeptides. |
|
|
Silk fibroin |
Consists of short amino acid side chains that assemble into β-sheet structures. |
SFs are sturdy, lightweight, and have exceptional strength and elasticity. Osteoconductivity. Biocompatible. Deliver good support for cell adhesion and proliferation without initiating cell toxicity. Promote cell migration and vascularization. Moderately degradable. Thermostable (up to ∼250 °C). Commonly employed as a cell carrier for cell seeding on scaffolds. |
Prolonged presence of silk may induce degradation, which releases certain residues or degraded products that may prompt the immune response. |
|||
|
Fibrinogen and fibrin |
Fibrinogen: Dimer consisting of three pairs of polypeptide chains (Aα, Bβ, and γ) |
Biocompatibility. High affinity for biological surfaces and molecules. Promotes cellular interactions. Variety of cell-adhesive/binding properties. Nonimmunogenicity. |
Low mechanical strength. Quick rate of degradation. |
|||
|
Gelatin |
Contains glycine residues, proline, and 4-hydroxyproline residues |
Better infiltration, adhesion, spreading, and proliferation of cells on resulting scaffolds. Good stability at high temperature in a broad range of pH. Biodegradability. Osteoconductivity. Non-immunogenic. Low antigenicity. |
Bioactivity is questionable in higher-order gelatin structures in scaffolds. Low stability in physiological conditions. |
|||
|
Keratin |
It is a cysteine-rich fibrous protein that associates with intermediate filaments (IFs) forming the bulk of the cytoskeleton and epidermal appendageal structures |
Facilitates cell adhesion and proliferation. Unique chemistry afforded by high sulfur content. Propensity for self-assembly. Intrinsic cellular recognition. Intrinsic biological activity. Cytocompatibility. Gradual degradation. |
Poor mechanical properties. Quick loss of mechanical integrity. |
|||
|
Polysaccharide-based scaffolds |
Starch |
Comprised of carbohydrates. The structure consists of two types of alpha glucan which are amylose and amylopectin. |
Biocompatible. Thermoplastic behavior. Non-cytotoxic. Guides various developmental stages of cells. Hydrophilicity. Good substrate for cell adhesion. Good biodegradation period. |
Very high water uptake. Low mechanical strength. Unstable for long-term application. Chemical modifications may lead to toxic byproducts and reduce the rate of degradation. |
||
|
Chitin/chitosan |
Chitin: N-acetyl glucosamine and N-glucosamine monomers Chitosan: N-deacetylated derivative of chitin |
Accelerates tissue repair. Prevents formation of scar tissue. Promotes cell adhesion. Non-toxic and non-allergenic. Bioactivity. Anti-inflammatory. Osteoconductivity. Hemostatic potential. Scaffolds could be used for a longer period. Chitosan-based scaffolds can immobilize growth factors. |
Poor mechanical strength and stability. High viscosity and low solubility at neutral pH. Rapid in vivo degradation rate. |
|||
|
Agarose |
Contains repeating units of agarobiose (a disaccharide of D-galactose and 3,6-anhydro-l-galactopyranose). |
Excellent biocompatibility. Thermo-reversible gelation behavior. Exceptional electroresponsiveness. Suitable medium for cell encapsulation. Non-immunogenic. |
Low cell adhesion. Nondegradability due to the absence of an appropriate enzyme in the body. |
|||
|
Alginate |
Made up of mannuronate and gluronate monomers. Different block configurations give rise to different materials properties. Mainly made up of carboxyl groups. |
Mimicking function of the extracellular matrix of body tissue. Thickening/gel-forming agent. Biocompatibility. Cytocompatibility. Biodegradability. Bioabsorbable. Hydrophilicity. |
Difficult to sterilize. Low cell adhesion. Poor mechanical characteristics. |
|||
|
Cellulose |
Polysaccharides are formed by many D-glucose units connected by glycosidic bonds. |
Stable matrix for tissue engineering applications. Better mechanical strength. Hydrophilicity. Biocompatibility. Cytocompatibility. Bioactivity. |
Cellulose in the human organism behaves as a nondegradable or very slowly degradable material. |
|||
|
Hyaluronic acid |
It is a linear, anionic, non-sulfated glycosaminoglycan with a structure composed of repeating disaccharides units: β-1,4-D-glucuronic acid and β-1,3-N-acetyl-D-glucosamide. |
Encapsulation capability. Cell activity. HA scaffolds are frequently used in the case of both hard and soft tissue regeneration. Nonimmunogenic. Nonantigenic. Biocompatibility. Osteocompatibility. |
Brittle; mechanical properties need fine-tuning via chemical modification. Low biodegradability in the crystalline phase. |
|||
|
Glycosaminoglycans |
Consist of repeating disaccharides linked by glycosidic bonds creating individual complex structures. |
Biocompatibility. Anticoagulant activity. Antithrombotic activity. Anti-inflammatory. Have multiple regulatory functions, e.g., in the anticoagulation of blood, inhibition of tumor growth, and metastasis. Control the inflammatory processes. |
Very fast degradation. Potential risk of contamination with infectious agents. |
|||
|
Synthetic polymers |
Poly(ƹ-caprolactone) (PCL) |
Aliphatic semicrystalline polyester. |
Controls cell proliferation and angiogenesis. Slow degradation rate (lower than that of PLA and PLGA). Non-toxic. Cytocompatibility. Good mechanical properties. Degraded by hydrolysis or bulk erosion. |
Low bioactivity. Hydrophobicity of PCL is another major issue that hinders wound healing application. Some problems related to withstanding mechanical loads. |
||
|
Polylactic acid (PLA) |
Highly crystalline. |
Biocompatible. Cytocompatibility. Thermal stability. Excellent mechanical strength. Good degradation rate. Nontoxic degradation products. |
PLA-based materials suffer from the lack of ideal surface chemistry that could aid cell adhesion and proliferation. Brittleness. Poor thermal stability. Hydrophobicity. |
|||
|
Polylactic-co-glycolic acid (PLGA) |
The copolymer of hydrophobic PLA and hydrophilic PGA. |
Excellent cell adhesion and proliferation. Good mechanical properties. Features faster degradation than either PGA or PLA. Wide range of degradation rates. |
Poor osteoconductivity. May develop biocompatibility problems. |
[159] |
||
|
Polyglycolic acid (PGA) |
Linear highly crystalline aliphatic polyester. |
Biocompatible. High tensile modulus. High melting point. Undergoes bulk degradation. Hydrophilicity. |
High sensitivity to hydrolysis. Difficult to obtain porous PGA scaffolds without toxic solvents. |
|||
|
Polyhydroxybutyrate (PHB) |
It is a homopolymer having a stereoregular structure with high crystallinity. Naturally occurring b-hydroxy acid. |
Non-toxic. Biostable. Biocompatible. Advantages over PLA and PGA. Slow rate of degradation. Can be obtained naturally. |
Inherent brittleness and rigidity. Thermal instability during melt processing impedes its commercial application. |
|||
|
Polypropylene fumarate (PPF) |
Linear and unsaturated copolyester based on fumaric acid. |
Biocompatibility. Crosslinked PPF matrices have high mechanical strength. PPF degrades in the presence of water into propylene glycol and fumaric acid, the degradation products that are easily cleared from the human body by normal metabolic processes. Non-toxic. |
It is a viscous liquid at room temperature (21 °C), making the handling of the polymer somewhat cumbersome |
|||
|
Poly(ethylene glycol) (PEG) |
Synthesized using ring-opening polymerization of ethylene oxide. |
Non-ionic. Biocompatible. Elasticity. Bioadhesive. Mucoadhesive. Hinders protein adsorption. Hydrophilic. PEG as a blank template can be modified to different moieties to pass different requirements of a skin substitute like cell adhesion, short-term degradation, and minimum inflammation. Non-immunogenic. |
Lacks cell-interactive character due to its bio-inert nature. Nonreactive, creates insoluble networks. |
|||
|
Polyurethane (PU) |
Urethane groups are the major repeating units. Synthesized by reactions of di- or polyisocyanates (hard segments) with di- or polyols (soft segments) via the catalyzed polymerization process. |
Bio- and hemocompatibility. Nontoxic. Biodegradable. Non-allergenic. Non-sensitizing. Excellent mechanical properties. High flexural endurance and fatigue resistance. |
PUs are less compatible with blood and found unsuitable for in vivo drug delivery application. Limited stability in vivo. |
|||
|
Polyvinyl alcohol (PVA) |
Semicrystalline polyhydroxy polymer. Prepared via hydrolysis of poly(vinyl acetate). |
Biocompatible. Nontoxic. Noncarcinogenic. Displays a reduced protein-binding tendency, relatively higher elasticity and water content; a highly hydrated water-soluble synthetic polymer. Has relatively similar tensile strength to human articular cartilages. Good lubrication. |
Lack of cell-adhesive property. Less ingrowth of bone cells. |
|||
|
Polypropylene carbonate (PPC) |
Product of alternating copolymerization of propylene oxide and CO2. Amorphous. |
Biodegradable amorphous polymer because of the aliphatic polycarbonate ester structure on its backbone. No inflammatory response. Thermoplastic behavior. Biocompatibility. Impact resistance. |
PPC has shortcomings such as viscous flow at room temperature and a relatively large brittleness at low temperature. Poor thermal and processing properties. Cell attachment to PPC is very limited due to its highly hydrophobic nature. |
|||
4. Final Remarks
In biomedical applications, scaffolds can be used ranging from regenerative engineering to controlled drug delivery and immunomodulation, and for this purpose, biomaterials have become an indispensable instrument as scaffold material. The materials used for scaffold manufacturing must satisfy some criteria such as intrinsic biofunctionality and appropriate chemistry to stimulate molecular biorecognition by cells to induce proliferation, cell adhesion, and activation. The mechanical properties of scaffolds and kinetics of decomposition in selected materials must be adjusted to the TE application specifically to ensure the essential structural functions and to achieve the rate of formation of new tissues. The geometrical features like exposed surface area, pore distribution and porosity, and distribution affect the rate of cell penetration within the scaffold volume, the architecture of the ECM formed. The final effectiveness of the regenerative process plays a major role in scaffolding. Many biodegradable polymers of natural and synthetic origin have been established for use as biomaterials and careful consideration of the cellular environment and interactions needed is required to select a polymer for a given application.
This entry is adapted from the peer-reviewed paper 10.3390/polym13071105
References
- Berthiaume, F.; Yarmush, M.L. Tissue Engineering. In Encyclopedia of Physical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 817–842.
- Furth, M.E.; Atala, A. Tissue Engineering. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 83–123.
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40.
- Bonassar, L.J.; Vacanti, C.A. Tissue Engineering: The First Decade and Beyond. J. Cell. Biochem. 1998, 30–31, 297–303.
- Bell, E. Tissue Engineering, An Overview. In Tissue Engineering; Bell, E., Ed.; Birkhäuser: Boston, MA, USA, 1993; pp. 3–15.
- Ude, C.C.; Miskon, A.; Idrus, R.B.H.; Abu Bakar, M.B. Application of Stem Cells in Tissue Engineering for Defense Medicine. Mil. Med. Res. 2018, 5, 7.
- Solchaga, L.A.; Goldberg, V.M.; Caplan, A.I. Cartilage Regeneration Using Principles of Tissue Engineering. Clin. Orthop. Relat. Res. 2001, 391, S161–S170.
- Ikada, Y. Challenges in Tissue Engineering. J. R. Soc. Interface 2006, 3, 589–601.
- Bianco, P.; Robey, P.G. Stem Cells in Tissue Engineering. Nature 2001, 414, 118–121.
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue Engineering: Strategies, Stem Cells and Scaffolds. J. Anat. 2008, 213, 66–72.
- Ripamonti, U. Soluble, Insoluble and Geometric Signals Sculpt the Architecture of Mineralized Tissues. J. Cell Mol. Med. 2004, 8, 169–180.
- Giardino, R.; Nicoli Aldini, N.; Torricelli, P.; Fini, M.; Giavaresi, G.; Rocca, M.; Martini, L. A Resorbable Biomaterial Shaped as a Tubular Chamber and Containing Stem Cells: A Pilot Study on Artificial Bone Regeneration. Int. J. Artif. Organs 2000, 23, 331–337.
- Ma, P.X. Scaffolds for Tissue Fabrication. Mater. Today 2004, 7, 30–40.
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926.
- Stock, U.A.; Vacanti, J.P. Tissue Engineering: Current State and Prospects. Annu. Rev. Med. 2001, 52, 443–451.
- Di Nardo, P.; Minieri, M.; Ahluwalia, A. Engineering the Stem Cell Niche and the Differentiative Micro- and Macroenvironment: Technologies and Tools for Applying Biochemical, Physical and Structural Stimuli and Their Effects on Stem Cells. In Stem Cell Engineering; Artmann, G.M., Minger, S., Hescheler, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 41–59.
- Liao, S.; Chan, C.K.; Ramakrishna, S. Stem Cells and Biomimetic Materials Strategies for Tissue Engineering. Mater. Sci. Eng. C 2008, 28, 1189–1202.
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic Polymer Scaffolds for Tissue Engineering. Chem. Soc. Rev. 2009, 38, 1139.
- Langer, R.; Tirrell, D.A. Designing Materials for Biology and Medicine. Nature 2004, 428, 487–492.
- Boccaccini, A.R.; Blaker, J.J. Bioactive Composite Materials for Tissue Engineering Scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317.
- Sultana, N. Biodegradable Polymer-Based Scaffolds for Bone Tissue Engineering; SpringerBriefs in Applied Sciences and Technology; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-34801-3.
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636.
- Hutmacher, D.W. Scaffolds in Tissue Engineering Bone and Cartilage. Biomaterials 2000, 21, 2529–2543.
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable Polymer Scaffolds for Tissue Engineering. Biotechnology 1994, 12, 689–693.
- Olivares, A.L.; Lacroix, D. Computational Methods in the Modeling of Scaffolds for Tissue Engineering. In Computational Modeling in Tissue Engineering; Geris, L., Ed.; Studies in Mechanobiology, Tissue Engineering and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2012; Volume 10, pp. 107–126.
- Giannitelli, S.M.; Accoto, D.; Trombetta, M.; Rainer, A. Current Trends in the Design of Scaffolds for Computer-Aided Tissue Engineering. Acta Biomater. 2014, 10, 580–594.
- Prasadh, S.; Wong, R.C.W. Unraveling the Mechanical Strength of Biomaterials Used as a Bone Scaffold in Oral and Maxillofacial Defects. Oral Sci. Int. 2018, 15, 48–55.
- Morouço, P.; Biscaia, S.; Viana, T.; Franco, M.; Malça, C.; Mateus, A.; Moura, C.; Ferreira, F.C.; Mitchell, G.; Alves, N.M. Fabrication of Poly(ε -Caprolactone) Scaffolds Reinforced with Cellulose Nanofibers, with and without the Addition of Hydroxyapatite Nanoparticles. BioMed Res. Int. 2016, 2016, 1–10.
- Yi, S.; Ding, F.; Gong, L.; Gu, X. Extracellular Matrix Scaffolds for Tissue Engineering and Regenerative Medicine. Curr. Stem Cell Res. Ther. 2017, 12, 233–246.
- Hosseinkhani, M.; Mehrabani, D.; Karimfar, M.H.; Bakhtiyari, S.; Manafi, A.; Shirazi, R. Tissue Engineered Scaffolds in Regenerative Medicine. World J. Plast. Surg. 2014, 3, 3–7.
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding Polymeric Biomaterials: Are Naturally Occurring Biological Macromolecules More Appropriate for Tissue Engineering? Int. J. Biol. Macromol. 2019, 134, 673–694.
- Mooney, D.J.; Sano, K.; Kaufmann, P.M.; Majahod, K.; Schloo, B.; Vacanti, J.P.; Langer, R. Long-Term Engraftment of Hepatocytes Transplanted on Biodegradable Polymer Sponges. J. Biomed. Mater. Res. 1997, 37, 413–420.
- Prakasam, M.; Popescu, M.; Piticescu, R.; Largeteau, A. Fabrication Methodologies of Biomimetic and Bioactive Scaffolds for Tissue Engineering Applications. In Scaffolds in Tissue Engineering—Materials, Technologies and Clinical Applications; Baino, F., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3641-5.
- Sanz-Herrera, J.A.; García-Aznar, J.M.; Doblaré, M. On Scaffold Designing for Bone Regeneration: A Computational Multiscale Approach. Acta Biomater. 2009, 5, 219–229.
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689.
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415.
- Ogueri, K.S.; Laurencin, C.T. Nanofiber Technology for Regenerative Engineering. ACS Nano 2020, 14, 9347–9363.
- Ogueri, K.S.; Jafari, T.; Escobar Ivirico, J.L.; Laurencin, C.T. Polymeric biomaterials for scaffold-based bone regenerative engineering. Regen. Eng. Transl. Med. 2019, 5, 128–154.
- Bhatia, S.K. (Ed.) Engineering Biomaterials for Regenerative Medicine; Springer: New York, NY, USA, 2012; ISBN 978-1-4614-1079-9.
- Lanza, R.P.; Langer, R.S.; Vacanti, J.; Atala, A. Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2020; ISBN 978-0-12-821401-5.
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A Review on Synthesis, Properties and Applications of Natural Polymer Based Carrageenan Blends and Composites. Int. J. Biol. Macromol. 2017, 96, 282–301.
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824.
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt Surg. 2018, 6, 90–99.
- Singh, M.R.; Patel, S.; Singh, D. Natural polymer-based hydrogels as scaffolds for tissue engineering. In Nanobiomaterials in Soft Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–260.
- Heidary Rouchi, A.; Mahdavi-Mazdeh, M. Regenerative Medicine in Organ and Tissue Transplantation: Shortly and Practically Achievable? Int. J. Organ. Transpl. Med. 2015, 6, 93–98.
- Zhu, J. Bioactive Modification of Poly(Ethylene Glycol) Hydrogels for Tissue Engineering. Biomaterials 2010, 31, 4639–4656.
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering Hydrogels as Extracellular Matrix Mimics. Nanomedicine 2010, 5, 469–484.
- Kluge, J.A.; Mauck, R.L. Synthetic/Biopolymer Nanofibrous Composites as Dynamic Tissue Engineering Scaffolds. In Biomedical Applications of Polymeric Nanofibers; Jayakumar, R., Nair, S., Eds.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2011; Volume 246, pp. 101–130.
- Cascone, M.G.; Barbani, N.; Cristallini, C.; Giusti, P.; Ciardelli, G.; Lazzeri, L. Bioartificial Polymeric Materials Based on Polysaccharides. J. Biomater. Sci. Polym. Ed. 2001, 12, 267–281.
- Jayakumar, R.; Nair, S. (Eds.) Biomedical Applications of Polymeric Nanofibers; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 246, ISBN 978-3-642-27147-2.
- Ciardelli, G.; Chiono, V.; Vozzi, G.; Pracella, M.; Ahluwalia, A.; Barbani, N.; Cristallini, C.; Giusti, P. Blends of Poly-(Epsilon-Caprolactone) and Polysaccharides in Tissue Engineering Applications. Biomacromolecules 2005, 6, 1961–1976.
