Cardiovascular diseases (CVDs) are the world’s leading cause of mortality and represent a large contributor to the costs of medical care. Although tremendous progress has been made for the diagnosis of CVDs, there is an important need for more effective early diagnosis and the design of novel diagnostic methods. The diagnosis of CVDs generally relies on signs and symptoms depending on molecular imaging (MI) or on CVD-associated biomarkers. For early-stage CVDs, however, the reliability, specificity, and accuracy of the analysis is still problematic. Because of their unique chemical and physical properties, nanomaterial systems have been recognized as potential candidates to enhance the functional use of diagnostic instruments. Nanomaterials such as gold nanoparticles, carbon nanotubes, quantum dots, lipids, and polymeric nanoparticles represent novel sources to target CVDs. The special properties of nanomaterials including surface energy and topographies actively enhance the cellular response within CVDs. The availability of newly advanced techniques in nanomaterial science opens new avenues for the targeting of CVDs.
- nanotechnology
- diagnosis
- treatment
- cardiovascular diseases
1. Introduction
Cardiovascular diseases (CVDs) are considered a major cause of mortality worldwide. According to the World Health Organization, CVDs led to the death of about 17.9 million people in 2016, contributing to 31% of total global deaths [1]. CVDs represent an economic burden for low- and middle-income countries as many people die at young age in their active time [2]. The heart consists of a variety of cells including cardiac fibroblasts, cardiomyocytes, neural cells, and vascular cells. Any dysfunction or abnormality in these endothelial, smooth, or connective cells results in CVDs [3]. The term CVDs encompasses a diverse set of disorders related to the heart which include ischemic heart disease, cerebrovascular diseases, arrhythmia, Marfan syndrome, thrombosis, pericardial disease, heart failure, stroke, vascular diseases, and cardiomyopathies [4]. Tobacco and alcohol consumption, unhealthy lifestyle, and genetic factors are the most possible risk factors to develop CVDs. Similarly, elevated blood glucose levels, obesity, and high lipid levels contribute to secondary underlying risk factors contributing to the progression of CVDs [5]. Besides the apparent pathophysiology, a variety of clinical investigations predicted that tumor necrosis factor-alpha (TNF-α) plays a key role in the pathogenesis of cardiac diseases. Cardiac monocytes and macrophages induce biochemical alterations along with compromised calcium metabolism. A decreased expression of β-adrenergic receptors, alterations in the natriuretic hormones, and increased matrix metalloproteinase (MMP) expression are also underlying risk factors [6].
Current treatment differs, depending on the risk and severity of CVDs. The main focus of all treatment protocols for CVDs is to improve the blood supply or prevent the pressure being exerted on cardiac walls in order to minimize tissue damage and capillaries rupture. Statin therapy is the most common option to dissolve the clots and regain endothelial membrane elasticity in blood vessels. Moreover, aspirin is the most frequently used drug for the secondary prevention of CVDs [7]. Alternatively, β-adrenergic receptor blockers are also prescribed as first-choice treatments for atrial fibrillation and coronary artery disease but they have been excluded from first-line therapy in the case of hypertension [8]. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin-II receptor blockers (ARBs) are also prescribed for the treatment of hypertension, congestive heart failure, and myocardial infarction [9]. Apart from conventional renin-angiotensin antagonists, new candidates like sacubitril/valsartan (an angiotensin receptor-neprilysin inhibitor) were investigated as more promising drugs to reduce the mortality in patients with cardiac failure with low ejection fraction, but clinical trials are still ongoing [10]. On the other hand, severe and chronic cases need highly invasive interventions like coronary artery bypass grafts, percutaneous transluminal coronary angioplasty, and stenting [11].
Right now, the most frequently used CVD diagnostic methods include magnetic resonance imaging (MRI), X-ray computed tomography (CT), electrocardiography (ECG), and echocardiography [12]. MRI employs radio waves to generate a 3D image of the heart and is most commonly used to diagnose stroke and atherosclerosis. CT uses X-rays beams to develop an image of the tissues with its high signal contrast. ECG records the electrical activity of the heart and monitors chest pain in patients with angina, heart attack, and arrhythmia. Echocardiography uses sound waves to create an image of the heart to detect cardiac disorders [13][14][15][16]. Additionally, advanced molecular imaging (MI) and cardiac immunoassays (CIAs) are new diagnostic methods to identify cardiac biomarkers (MMP-1, hepatocyte growth factor, cyclooxygenase 2, monocyte chemoattractant protein 1—MCP-1, tissue inhibitor of MMP-1). MI has been merged with various other techniques over the past to acquire more reliable and sensitive outcomes with accuracy [17][18].
Despite ongoing progress in pharmacological and clinical treatment, CVDs are still the leading cause of morbidity and mortality worldwide. The current treatments are not disease-specific and generally lead to organ toxicity. The blood thinning agents and anti-coagulants may pose serious adverse effects to the heart and limit the optimal dose administration, which itself remains a serious therapeutic challenge [19]. Aspirin is reported to have an increased risk of bleeding, therefore, international guidelines on primary prevention of CVDs recommend aspirin only if the risk of cardiac events persist for at least 10 years [20]. Likewise, animal studies revealed that implantation of stents results in irritation, allergic reactions, and other significant issues like webbing, causing thrombogenicity [21]. The most commonly employed X-ray techniques have short imaging window and are limited in the contrast and differentiation of soft tissues, healthy tissues, and pathological tissues. Also, the diagnosis of CVDs using conventional techniques lacks precision, accuracy, and early diagnosis because of heterogeneity of CVDs [22][23].
Nanomedicine is a convergent discipline that combines the fields of biology, biochemistry, physics, engineering, genetics, and biotechnology to adjust the treatment and diagnosis of diseases [24][25][26]. Chronic heart diseases, especially myocardial infarction, eventually end up with a heart transplant, which may not be feasible, usually because of the unavailability of donors, autoimmune disorders, and chances of organ rejection. Recently, biomimetic materials based on nanotechnology gained increased attention for the development of scaffolds [27]. These scaffolds are composed of nanomaterials to provide mechanical, electromagnetic, and physical assistance in tissue repair and regeneration. They can provide cells via seeding at a site of injury or tissue deterioration to aid tissue functioning and healing [28]. Presently, nanopolymeric-coated biodegradable stents are being explored to resolve the issues with the conventional stents in use. These novel stents can improve drug release profiles along with a reduction in the platelet adhesion rate. Nanocomposite polymers including poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and polyhedral oligomeric silsesquioxane poly-(carbonate-urea) (POSS-PCU) are being used to construct anti-thrombogenic and blood compatible stents [29][30][31]. Nanomedicines of different characteristics and compositions are under extensive research for the treatment of CVDs. These include polymeric nanoparticles (NPs), silica-based nanoconjugates, micelles, liposomes, niosomes, exosomes, surface-modified nanostructures, nanofibers, nanotubes, metallic NPs, dendrimers, hybrid nanosystems, poly(ethylene glycol)-ated (PEGylated) nanospheres, and immunomodified nanoshells [32][33][34][35][36], as presented in Figure 1.
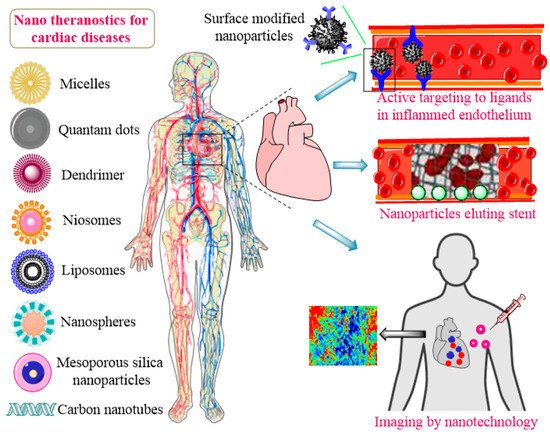
Figure 1. Application of nanotheranostics for CVDs.
CVDs may be treated if accurately diagnosed at early stages. Recent trends in nanotechnology also facilitated the diagnosis of CVDs. Nanoscale contrast agents emerged as multifaceted entities that can diagnose cardiac disorders in the very initial stages. Nanosensors are advantageous in contrast to conventional diagnostic approaches because they can incorporate a variety of imaging agents and simultaneously be used to load drugs for active targeting. They allow for a chelation of multivalent targeting moieties due to increased surface areas. Contrast-enhancing nanostructures for diagnosis and imaging comprise of fluorescent, paramagnetic, multimodal, light scattering, electron-dense, or radioactive particles, collectively termed as nanosensors [37][38]. Nanosensors provide clinical accuracy and usefulness by the detection of even single nucleotide polymorphisms (SNPs). Moreover, advancements in diagnosis and imaging using nanosensors further improved the detection of proteins and biomarkers in CVDs [39]. Nanosensors and nanomaterials allow for a local or targeted delivery and diagnosis, reduced sheer impact of blood flow, reduction in dose, and prolonged effects [40][41][42]. Disease-specific molecules or proteins can be targeted for treatment approaches by nanomaterials or quantitatively estimated by nanosensors, e.g., specific integrins (ανβ3-integrin, vascular cell adhesion molecule 1-VCAM-1) expressed by atherosclerotic plaques can be exploited as target [43]. Emerging diagnostic tools like in vitro programmable bionanochips (lab-on-chip) are in demand for point-of-care testing [44]. Another advancement in the diagnosis of pressure-related diseases is the pressure biosensor. Such a biosensor uses self-oriented nanocrystals in spatial arrangements to capture micropressure changes on cardiovascular walls. These nanosensors hold optimistic results in the postoperative recrudescence of deep thrombus [45]. Moreover, various supraparamagnetic iron oxide NPs (SPIONs) and ultrasmall SPIONs (USPIONs) are showing promising results as contrast agents in clinical trials for CVDs. As macromolecules are unable to cross cellular biological barriers and have limited availability at diseased sites, targeted drug, and biologic delivery, along with diagnosis of the cardiac tissues and organelles which need to be further exploited for a rational treatment of CVDs.
2. Applications of Nanomaterials in the Treatment of CVDs
Nanomaterials also play significant roles in the treatment of CVDs [46]. Nanomaterial applications are closely related to the medical research, providing strong alternatives for CVDs. Many new strategies may enhance the efficacy of these treatments. Progress has been made in the past decades in the application of nanotechnology and nanomaterials for CVDs [47][48][49][50][51][52][53][54][55][56][57]. NPs are considered as safe and efficacious platforms for different drugs whose clinical utilities are limited due to their toxicity or unfavorable pharmacokinetic properties. Figure 2 presents common nanomaterials used in CVD application and their common features [40].
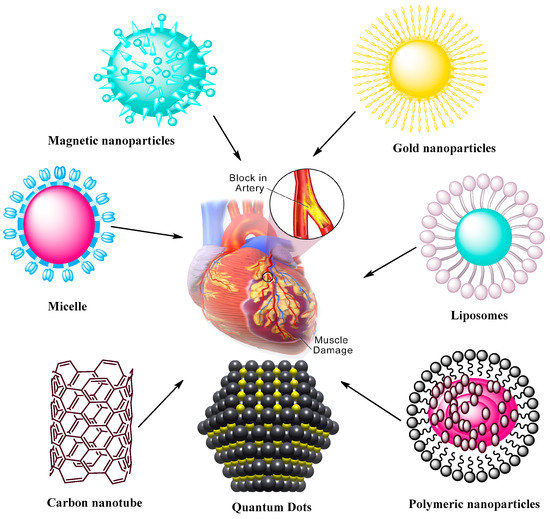
Figure 2. Nanoparticle-mediated drug delivery systems to target CVDs. The systems include magnetic and AuNPs, self-assembling micelles, liposomes, carbon nanotubes, quantum dots, and polymeric NPs formed via assembly of polymers containing therapeutic agents.
2.1. Liposomes
There is much interest in developing lipid-based drug delivery systems such as solid lipid NPs, liposomes, and self-emulsifying systems to target CVDs. The studies demonstrated that drugs encapsulated in liposomes or in lipid nanocarrier systems can lower the toxicity issues compared with free drug intervention in CVDs. Lipid-based systems have been explored to deliver biomolecules for vascular abnormalities [58].
Dzau and coworkers [59] showed the efficacy of fusigenic lipids for gene therapy of CVDs. Another study encapsulated DNA in liposomes with an average diameter of 200 nm where the lipid membrane was modified with proteins of UV-inactivated hem-agglutinating virus [60]. The liposomes attached to plasma membranes within a short time, allowing for cytostatic gene therapy in models of vascular proliferative diseases. Electrostatic DNA complex with cationic liposomes has also been explored for vascular disease gene therapy. In some studies, antibodies attached to liposomes were utilized to actively target fibrinogen, intercellular adhesion molecule-1, and fibrin. This type of liposomes provides a method for the use of ultrasound-induced cavitation to transport fibrinolytic agent. Dasa and coworkers [61][62] developed peptide liposomal systems with an affinity for cell types present in postmyocardium infarction. They encapsulated the poly(ADP ribose) polymerase-1 inhibitor AZ7379 in a liposomal system and measured increased bioavailability of AZ7379 at infarct zone 24 h after injection compared with non-liposomal AZ7379. Lestini et al. [63] functionalized liposomes using arginine-glycine-aspartic acid (RGD) to target integrin GPIIb-IIIa receptors on activated platelets. Moreover, surfactant oil godextran incorporation in the liposomes provided knowledge into the effects of vesicle perturbation on liposome clearance. This ligand-gated delivery of liposomes highlights the importance of selective targeting in design of effective targeted delivery system in CVD treatment. van der Valk et al. [64] manipulated liposome-based drug delivery to improve the risk/benefit ratios of active agents to target atherosclerotic plaques as the very 1st clinical study of prednisolone-loaded liposomes (PLPs) against atherosclerosis. When tested in humans, PLPs build on pharmacokinetic profiles with increased plasma half-life of 63 h. Moreover, intravenously infused liposomal NP PLPs (LN-PLPs) appeared in 75% of the macrophages. The study data concluded on the successful delivery of long circulating liposomes to atherosclerotic plaque macrophages in patients, showing the value of the approach for the development and imaging-assisted evaluation of future nanomedicines in atherosclerosis [64]. Laing et al. [65] evaluated the thrombolytic effects of tissue plasminogen activator (tPA) encapsulated in echogenic liposomes (ELIPs) in an in vivo rabbit aorta clot model. Thrombus was studied in the abdominal aorta and the etiology was imaged before ELIP administration while blood flow velocities were measured before and after treatment. Ultrasound treatment enhanced the thrombolytic effect of tPA-loaded ELIPs with complete recanalization rates versus ineffective empty ELIPs, highlighting the effective thrombolytic effects of tPA in vivo via ELIP loading, while Doppler treatment increased the thrombolytic effects with enhanced and rapid recanalization rates. Zhong et al. [66] manipulated the bioactive flavonoid substance breviscapine obtained from traditional Chinese medicine against CVDs. This study used multivesicular liposomes (MVLs) as sustained release system to reduce the frequency of injection and afford patient compliance. Pharmacokinetics study were performed using MVLs containing breviscapine relative to traditional liposomes upon intramuscular injection in rats. The results showed increased residence time and drug release in vitro while absorption in vivo showed good linear correlation, supporting the concept of using MVLs as a sustained delivery system of breviscapine for CVDs. Huang et al. [67] worked on an injectable delivery system that can selectively drive thrombolytics to sites of active platelet aggregation, having significant potential for vascular occlusion therapy. The authors performed surface modification with a conformationally high affinity RGD motif for GPIIb-IIIa-binding. The targeting/binding of RGD-modified liposomes was studied by scanning electron microscopy, fluorescence, and flow cytometry and the attachment of the RGD liposomes was evaluated in a rat model and monitored ex vivo by fluorescence microscopy. The results revealed the feasibility of changing the binding potential of vascularly targeted liposomes and platelet targeting using surface-modifying ligands. Figure 3 presents functionalized liposomes for targeting the CVDs.
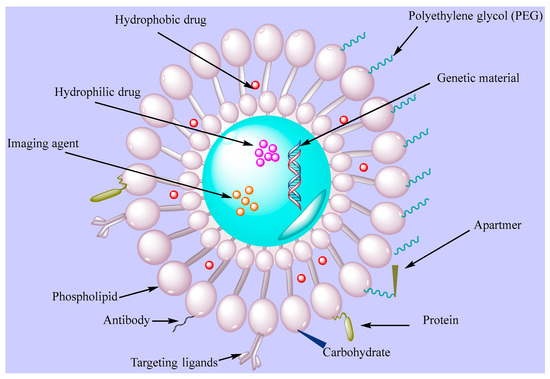
Figure 3. Functionalized liposomes with targeting ligands (PEG, antibodie, apartmers, proteins, carbohydrates).
2.2. Polymeric Nanoparticles
The tunable properties of polymeric NPs are the main focus of CVD treatment, having a potential for reabsorption in the body. Polymeric NPs can be coupled or free floating with another material [68].
Research was performed to immobilize PLGA on a polytetrafluoroethylene film as a material to develop vascular grafts [69]. This approach may be used to deliver specific molecules such as antithrombotic drugs to act against the regular side effects of thrombosis. Ahadian et al. [70] developed polymeric nanomaterials for CVD applications using a matrix of polyester and carbon nanotubes (CNTs) with enhanced electrical conductivity and stability, allowing to stimulate cell-cell coupling. Matoba et al. [61] investigated polymeric PLGA NPs as a nanodrug delivery system to impact monocyte-derived inflammation during atherosclerosis. FTIC-loaded PLGA NPs were incorporated in mice after 2 h of plaque rupture, as noted by flow cytometric analysis of aortic and peripheral leukocytes, with delivery of FITC NPs to peripheral monocytes and next to aortic macrophages 7 days after injection. This group also developed PLGA NPs encapsulated with pioglitazone, a clinically approved thiazolidinedione that activates the peroxisome proliferator activated receptor-gamma (PPARγ), allowing to regulate inflammation in mice in vivo for up to 4 weeks. Pioglitazone NPs significantly reduced the number of fibrous caps and enhanced the fibrous cap thickness, but orally administered pioglitazone did not affect the number of fibrous caps thickness or plaque area, showing the efficacy of PLGA NPs as delivery system. Figure 4 presents nanodrug delivery systems mediated treatment for acute coronary syndrome.
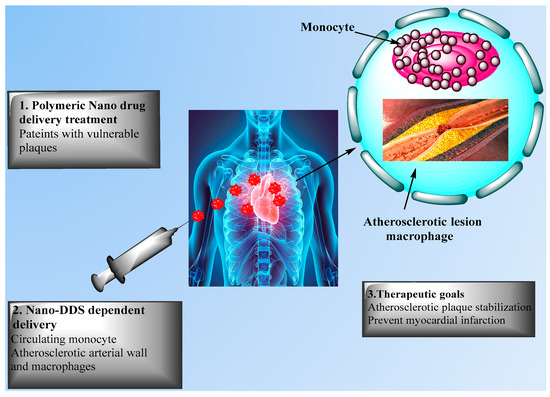
Figure 4. Nanodrug delivery systems for the treatment of acute coronary syndrome. The Figure presents nanodrug delivery system (Nano-DDS)-mediated treatments for patients with unstable plaque via (1) intravenous injection and (2) delivery to circulating monocytes, with (3) therapeutic goals including atherosclerotic plaque stabilization and prevention of acute myocardial infarction.
2.3. Micelles
Micelles are specialized, efficient carriers for poorly soluble drugs as nanodimensional colloidal particles with a hydrophilic shell and hydrophobic core. Surface-modified polymeric micelles primarily consist of amphiphilic macromolecules and have many applications in the areas of drug delivery and theranostics.
Various polysaccharides have been employed to prepare polymeric micelles. PEGylated polycationic block copolymeric micellar assembly was established to target vascular injury in the rabbit carotid artery for gene delivery [71]. Peters et al. [72] demonstrated that a PEG-based lipid micelle system co-encapsulated with a fluorophore as an imaging agent was capable of delivering an anticoagulant drug at the similar targeting site. Other studies demonstrated the efficacy of PEG-based lipid micelles with surface-modified scavenger receptor-based antibodies and encapsulated with gadolinium (Gd) complex to particularly accumulate in atherosclerotic arterial sites. At atherosclerotic aortic sites, Gd-encapsulated micelles functionalized with anti-CD36 antibodies could reveal macrophages in human atherosclerotic aortic tissues obtained at autopsy. Ding and colleagues [73][74] explored a number of probable micelle-based strategies intended for improperly functioning endothelia that forms a significant part of thrombotic or atherosclerotic tissues. Their results showed a significant potential of micellar site-specific delivery and usefulness for the treatment of CVDs. Wennink et al. [71] worked on the targeted elimination of macrophages by photodynamic therapy as a promising approach to reduce atherosclerotic plaques. Temoporfin or m-tetra(hydroxyphenyl)chlorin (mTHPC) may be suitable as photosensitizer for application in atherosclerotic lesions and cancer. In this particular study, mTHPC was loaded in polymeric micelles developed by the film hydration method using benzyl-PCL-b-methoxy PEG. The authors reported that delivery of mTHPC in blood plasma from the micelles occurred after 30 min and that accumulation of mTHPC in atherosclerotic lesions of mice resulted in binding to lipoproteins upon release from the micelles. Future experiments may allow to increase the stability and accumulation of THPC-loaded Ben-PCL-mPEG micelles to macrophages of atherosclerotic lesions. Yoo et al. [75] developed fibrin-binding, peptide amphiphile micelles (PAMs) by encapsulation the targeting peptide cysteine-arginine-glutamic acid lysine alanine (CREKA) with two types of amphiphilic molecules containing Gd, the chelator diethylenetriaminepentaacetic acid (DTPA) as DTPA-bis(stearyl amide) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(PEG)-2000)-DTPA as (DSPE-PEG2000-DTPA). An atherosclerotic mouse model was evaluated for clot-binding properties in vitro, contrast enhancement, and safety via magnetic and optical imaging. The results of in vivo imaging and optical studies of the aortas and heart showed the fibrin specificity conferred by the peptide ligand. Biodistribution studies confirmed that all micelles were cleared via renal clearance and the reticuloendothelial system. These studies revealed the successful molecular imaging in vitro vs. in vivo for site-specificity and provided a platform for the detection of thrombosis via contrast-enhancing agents in theranostic applications. Kirana et al. [76] evaluated the efficacy of artificially developed micelles using naturally developed micelles from pig bile to assess the potential of inhibitors targeting cholesterol uptake in cultured Caco-2 cells. Overall, pig bile was a convenient and cost-effective source of micelles for cellular uptake and cholesterol micelle solubility assays that was also efficient to screen potential cholesterol lowering agents.
2.4. Magnetic Nanoparticles
During the last decades, magnetic NPs have been used at large scale in biomedical applications, including as contrast agents in immunoassays, MRI, and cell sorting assays. Among them, iron oxide-based magnetic NPs are most commonly employed due to their lesser saturation magnetization, lower magnetic characteristics, and lower specific loss of power compared with cobalt (Co) iron (Fe) nanomaterials. NPs such as perfluorocarbon NPs and super magnetic NPs have been developed for MRI. These particles designed to target specific receptors or epitopes in tissues are near to enter clinical trials for CVD applications. As angiogenesis is the key part of atherosclerotic plaques, specific recognition of angiogenesis in early vascular diseases is not possible. However, paramagnetic NPs can spatially locate and measure atherosclerotic plaques in hyperlipidemic rabbits [77][78]. In this study atherosclerotic rabbits were treated with αvβ3-targeted paramagnetic NPs including fumagillin and MRI signal assessments of neovascular proliferation within the aortic wall. Magnetic NPs have physical and biological properties with desirable size distribution, higher magnetic flux density with increasing penetrability, and an ability to translate magnetic waves to heat energy, being both highly biocompatible and non-toxic. SPIONs are among most used magnetic NPs and can serves as good nanotheranostics [79]. In biomedical applications, magnetic NPs are template particles (micelles, perfluorocarbon bubbles, lipid vesicles etc.) that may be modified with paramagnetic elements such as chelated Gd or particles of metals with greater magnetization such as iron and cobalt. In angiogenesis, integrin v3 is upregulated in the vascular endothelium, leading to the development of functionalized magnetic NPs with v3-targeting ligands decorated with Gd and the potential use of contrast-enhanced MRI for CVDs associated with angiogenic vasculature [80][81].
2.5. Gold Nanoparticles
AuNPs provide suitable carrier systems in the field of nanotechnology, especially to treat CVDs, due to their properties such as low cytotoxicity, stability, and biocompatibility. They have a broad level of applications in biomedical science including molecular labeling and drug delivery, with a significant stability in the circulatory system and ability to decompose in a proper environment once reaching the target site. The large surface area property of AuNPs allows their functionalization with a number of biological molecules (ligands) including drugs, proteins, antibodies, and genes.
Work is ongoing for the imaging of CVDs based on the use of AuNPs with applications in atherosclerotic lesions and inflammation [82]. Photothermal therapeutic strategies have been also developed using the properties of Au for the preparation of vascular drug-encapsulated nanostructures. The following reaction can assist in thermal instability of NPs to liberate the encapsulated drugs at the respective targeting site. Researchers are working to evaluate the role of macrophages in vascular lesions and their ability to uptake AuNPs to facilitate contrasting enhanced intravascular photoimaging of cardiovascular abnormalities. Moreover, AuNPs have also efficient antioxidant properties that may be usefult to manage CVDs. The photothermal property of AuNPs was employed for plaque specific delivery of such NPs for the diagnosis of photothermal revascularization of blocked arteries [83]. Ghann and coworkers [84] encapsulated lisinopril as an active agent to develop AuNP-based CT contrast agents. AuNP conjugated with pure lisinopril, reduced thioctic lisinopril, or thioctic lisinopril were developed through ligand exchange reaction on citrate-coated AuNPs. The higher stability properties of thioctic lisinopril AuNPs were used to evaluate the targeting of angiotensin converting enzyme (ACE) using X-ray CT. The images showed high contrast in the region of heart and lungs clearly indicating the targeting of ACE and overexpression of ACE was related to the development of pulmonary and cardiac fibrosis. This new strategy may serve as a useful tool to monitor cardiovascular pathophysiology using CT imaging. AuNPs used alone or in combination may offer large scale treatment and diagnosis approaches in CVDs.
2.6. Dendrimer Nanostructures
Dendrimer nanostructures exhibit tree-like architectures occurring via either the convergent method or divergent method. Polyamido-amines are most common dendrimers with nitric oxide (NO) as free radical for application in CVDs due to its prominent relaxing action on vascular smooth muscle [85][86]. siRNA-based oligo-arginine conjugated dendritic delivery systems were used to silence ATIR expression in cardiomyocytes in vitro, showing efficient storage of cardiac functions in case of ischemic reperfusion injury in rats [87][88]. Lysine-based dendrimer architectures were also employed against atherosclerosis, with dendritic assemblage in arterial walls resulting in the controlled release and therapeutic delivery of NO [89][90]. Biocompatible and functionalized dendritic structures with cyclic RGD peptide containing entrapped 76Be has been site-specifically applied for PET imaging in mice. Streptokinase-modified dendrimers synthesized with different types of polymers (PLGA or chitosan or PEG, glycol chitosan) were shown to sustain the delivery of anti-thrombotic agents in the circulation [78][89]. Figure 5 presents the general structure of dendrimers.
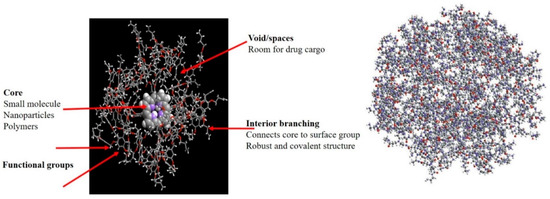
Figure 5. Schematic representation of dendrimers.
2.7. Carbon Nanotubes
The architectural assembly of CNTs can be described as molded or wrapped grapheme sheets. The defined structure of CNTs provides significant physiochemical properties, enhanced surface area, high optical properties, strong mechanical stability, and enhanced electrical conductivity.
Many studies reported the lack of toxicity of CNTs to deliver a variety of biological active molecules in vitro and in vivo for use in biomedical fields. For example, application of CNTs to cultured cardiomyocytes was shown to augment their viability and proliferation and to induce their maturation as reported when evaluating their electrophysiological features, while these systems can provide support to the endothelial cells in blood vessels for supplying oxygen to the heart muscle [91][92][93]. Garibaldi and coworkers [94] explored the potential of CNTs as biocompatible probes for applications in cardiomyocyte research. The authors reported that highly purified single walled CNTs had no toxic effects on the H9c2(2-1) rat cell line as seen by an estimation of cell proliferation and viability vs. apoptosis (tryptan blue exclusion, flow cytometry) and of morphological changes under light microscopy, supporting further work for CVD diagnosis and treatment.
2.8. Quantum Dots
QDs are nanocrystals semiconductor with an average diameter of 10 nm. They possess size-dependent fluorescent properties and display different quantized energy levels. As QDs have some limitations regarding the clinical safety since they were use heavy metals that might cause toxicity or other side effects, recent work was developed to prepare heavy metal-free QDs that are less toxic [95]. QDs of cadmium selenite encapsulated with zinc sulphide were developed over which polar polymeric biomolecules can be coated for targeted delivery as shown on Figure 6.
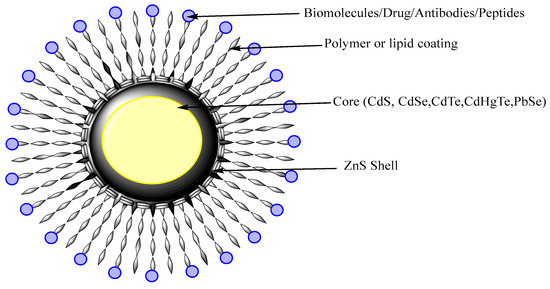
Figure 6. Schematic diagram of QDs. QDs have a core structure of heavy metal that provides fluorescence. The shell stabilizes the core and polymeric coating direct the targeted or active delivery.
Ferrara and coworkers [96][97] explored different possibilities of QDs for stimulation of cell adhesion molecules in targeting vascular diseases. In this purpose, QDs were functionalized with specific antibodies directed to target specific types of CVDs. Yan et al. [98] evaluated the vascular toxicity of mercapto-succinic acid-capped CdTe QDs in vitro. The authors showed that CdTe QDs dose-dependently reduced the viability of human umbilical vein endothelial cells (HUVECs), indicating CdTe QDs induced significant endothelial toxicity. The results of immunofluorescence and cytometric analyses revelaed that QDs elicited mitochondrial network fragmentation and significant oxidative stress along with a disruption of membrane potential, suggesting that exposure to QDs is of significant risk for the development of CVDs. Gilaizik et al. [99] worked on QDs and elaborated optical features with great potential as imaging tools compared with other traditional dyes, but again systemic toxicity limited their application in vivo. Vascular inflammation is one of the features that take part in CVDs such as poor prognosis and restenosis with enhanced numbers of monocyte-derived macrophages (MDMs). This study reported the structural stability, cellular uptake, physiochemical characteristics and biodistribution of MDM-targeted liposomal QDs (LipQDs) following local intra-luminal intervention of LipQDs in carotid injured rat relative to systemic intervention and imaging of QDs in the arterial tissues. Compared with free QDs, the LipQDs had versatile properties such as fluorescent stability, increased accumulation and retention for up to 24 h, and targeting delivery enabling MDM imaging that may serve for screening purposes in injured arteries.
This entry is adapted from the peer-reviewed paper 10.3390/chemosensors9040067
References
- WHO. Cardiovascular Diseases (CVDs). 2017. Available online: (accessed on 4 July 2018).
- Flora, G.D.; Nayak, M.K. A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr. Pharm. Des. 2019, 25, 4063–4084.
- Zamani, P.; Fereydouni, N.; Butler, A.E.; Navashenaq, J.G.; Sahebkar, A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc. Med. 2019, 29, 313–323.
- Braunwald, E. Cardiomyopathies: An overview. Circ. Res. 2017, 121, 711–721.
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808.
- Chiba, A.; Watanabe-Takano, H.; Miyazaki, T.; Mochizuki, N. Cardiomyokines from the heart. Cell. Mol. Life Sci. 2018, 75, 1349–1362.
- Putzu, A.; de Carvalho, C.M.P.D.; de Almeida, J.P.; Belletti, A.; Cassina, T.; Landoni, G.; Hajjar, L.A. Perioperative statin therapy in cardiac and non-cardiac surgery: A systematic review and meta-analysis of randomized controlled trials. Ann. Intensive Care 2018, 8, 95.
- Diaconu, C.C.; Marcu, D.R.; Bratu, O.G.; Stanescu, A.M.A.; Gheorghe, G.; Hlescu, A.A.; Mischianu, D.L.; Manea, M. Beta-blockers in cardiovascular therapy: A review. J. Mind Med. Sci. 2019, 6, 216–223.
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.-J.; Xie, J.; Liu, Y.-M.; Zhao, Y.-C.; Huang, X.; Lin, L. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020, 126, 1671–1681.
- Ambrosy, A.P.; Mentz, R.J.; Fiuzat, M.; Cleland, J.G.; Greene, S.J.; O’Connor, C.M.; Teerlink, J.R.; Zannad, F.; Solomon, S.D. The role of angiotensin receptor–neprilysin inhibitors in cardiovascular disease-existing evidence, knowledge gaps, and future directions. Eur. J. Heart Fail. 2018, 20, 963–972.
- Rodriguez-Araujo, G.; Krentz, A.J. Utility of invasive and non-invasive cardiovascular research. In Translational Research Methods in Diabetes, Obesity, and Nonalcoholic Fatty Liver Disease: A Focus on Early Phase Clinical Drug Development; Springer: Cham, Switzerland, 2019; pp. 275–308.
- Shi, C.; Xie, H.; Ma, Y.; Yang, Z.; Zhang, J. Nanoscale technologies in highly sensitive diagnosis of cardiovascular diseases. Front. Bioeng. Biotechnol. 2020, 8, 531.
- Morris, S.A.; Slesnick, T.C. Magnetic resonance imaging. In Visual Guide to Neonatal Cardiology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 104–108.
- Alsharqi, M.; Woodward, W.; Mumith, J.; Markham, D.; Upton, R.; Leeson, P. Artificial intelligence and echocardiography. Echo Res. Pract. 2018, 5, R115–R125.
- Picard, F.; Sayah, N.; Spagnoli, V.; Adjedj, J.; Varenne, O. Vasospastic angina: A literature review of current evidence. Arch. Cardiovasc. Dis. 2019, 112, 44–55.
- Schoepf, U.J. CT of the Heart; Humana Press: Totowa, NJ, USA, 2019.
- Oikonomou, E.K. Molecular Imaging to Guide Precision Diagnosis and Prevention of Cancer Therapeutics-Related Cardiac Dysfunction; Taylor & Francis: Abingdon, UK, 2020.
- Clerico, A.; Lippi, G. The state-of-the-art of “high-sensitivity” immunoassay for measuring cardiac troponin I and T. J. Lab. Precis. Med. 2018, 3, 53.
- Chandarana, M.; Curtis, A.; Hoskins, C. The use of nanotechnology in cardiovascular disease. Appl. Nanosci. 2018, 8, 1607–1619.
- Raber, I.; McCarthy, C.P.; Vaduganathan, M.; Bhatt, D.L.; Wood, D.A.; Cleland, J.G.; Blumenthal, R.S.; McEvoy, J.W. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet 2019, 393, 2155–2167.
- Univers, J.; Long, C.; Tonks, S.A.; Freeman, M.B. Systemic hypersensitivity reaction to endovascular stainless steel stent. J. Vasc. Surg. 2018, 67, 615–617.
- Bejarano, J.; Navarro-Marquez, M.; Morales-Zavala, F.; Morales, J.O.; Garcia-Carvajal, I.; Araya-Fuentes, E.; Flores, Y.; Verdejo, H.E.; Castro, P.F.; Lavandero, S. Nanoparticles for diagnosis and therapy of atherosclerosis and myocardial infarction: Evolution toward prospective theranostic approaches. Theranostics 2018, 8, 4710.
- Tian, L.; Lu, L.; Feng, J.; Melancon, M.P. Radiopaque nano and polymeric materials for atherosclerosis imaging, embolization and other catheterization procedures. Acta Pharm. Sin. B 2018, 8, 360–370.
- Richardson, J.J.; Caruso, F. Nanomedicine toward 2040. Nano Lett. 2020, 20, 1481–1482.
- Barani, M.; Torkzadeh-Mahani, M.; Mirzaei, M.; Nematollahi, M.H. Comprehensive evaluation of gene expression in negative and positive trigger-based targeting niosomes in HEK-293 cell line. Iran. J. Pharm. Res. IJPR 2020, 19, 166.
- Bilal, M.; Barani, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanomaterials for the treatment and diagnosis of Alzheimer’s disease: An overview. NanoImpact 2020, 20, 100251.
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int. J. Biol. Macromol. 2019, 140, 278–287.
- Mukhtar, M.; Ali, H.; Ahmed, N.; Munir, R.; Talib, S.; Khan, A.S.; Ambrus, R. Drug delivery to macrophages: A review of nano-therapeutics targeted approach for inflammatory disorders and cancer. Expert Opin. Drug Deliv. 2020, 17, 1239–1257.
- Sevostyanov, M.; Baikin, A.; Sergienko, K.; Shatova, L.; Kirsankin, A.; Baymler, I.; Shkirin, A.; Gudkov, S. Biodegradable stent coatings on the basis of PLGA polymers of different molecular mass, sustaining a steady release of the thrombolityc enzyme streptokinase. React. Funct. Polym. 2020, 150, 104550.
- Sun, J.; Sun, K.; Bai, K.; Chen, S.; Wang, F.; Zhao, F.; Hong, N.; Hu, H. A novel braided biodegradable stent for use in congenital heart disease: Short-term results in porcine iliac artery. J. Biomed. Mater. Res. Part A 2019, 107, 1667–1677.
- Omid, S.O.; Goudarzi, Z.; Kangarshahi, L.M.; Mokhtarzade, A.; Bahrami, F. Self-expanding stents based on shape memory alloys and shape memory polymers. J. Compos. Compd. 2020, 2, 92–98.
- Maleki, B.; Alinezhad, H.; Atharifar, H.; Tayebee, R.; Mofrad, A.V. One-pot synthesis of polyhydroquinolines catalyzed by ZnCl2 supported on nano Fe3O4@ SiO2. Org. Prep. Proced. Int. 2019, 51, 301–309.
- Cervadoro, A.; Palomba, R.; Vergaro, G.; Cecchi, R.; Menichetti, L.; Decuzzi, P.; Emdin, M.; Luin, S. Targeting inflammation with nanosized drug delivery platforms in cardiovascular diseases: Immune cell modulation in atherosclerosis. Front. Bioeng. Biotechnol. 2018, 6, 177.
- Sandoval-Yañez, C.; Castro Rodriguez, C. Dendrimers: Amazing platforms for bioactive molecule delivery systems. Materials 2020, 13, 570.
- Dizaj, S.M.; Rad, A.A.; Safaei, N.; Salatin, S.; Ahmadian, E.; Sharifi, S.; Vahed, S.Z.; Lotfipour, F.; Shahi, S. The application of nanomaterials in cardiovascular diseases: A review on drugs and devices. J. Pharm. Pharm. Sci. 2019, 22, 501–515.
- Thompson, L.C.; Sheehan, N.L.; Walters, D.M.; Lust, R.M.; Brown, J.M.; Wingard, C.J. Airway exposure to modified multi-walled carbon nanotubes perturbs cardiovascular adenosinergic signaling in mice. Cardiovasc. Toxicol. 2019, 19, 168–177.
- Giménez, V.M.M.; Fuentes, L.B.; Kassuha, D.E.; Manucha, W. Current Drug Nano-targeting Strategies for Improvement in the diagnosis and treatment of prevalent pathologies such as cardiovascular and renal diseases. Curr. Drug Targets 2019, 20, 1496–1504.
- Tu, Y.; Sun, Y.; Fan, Y.; Cheng, Z.; Yu, B. Multimodality molecular imaging of cardiovascular disease based on nanoprobes. Cell. Physiol. Biochem. 2018, 48, 1401–1415.
- Li, T.; Liang, W.; Xiao, X.; Qian, Y. Nanotechnology, an alternative with promising prospects and advantages for the treatment of cardiovascular diseases. Int. J. Nanomed. 2018, 13, 7349.
- Deng, Y.; Zhang, X.; Shen, H.; He, Q.; Wu, Z.; Liao, W.; Yuan, M. Application of the nano-drug delivery system in treatment of cardiovascular diseases. Front. Bioeng. Biotechnol. 2020, 7, 489.
- Su, M.; Dai, Q.; Chen, C.; Zeng, Y.; Chu, C.; Liu, G. Nano-medicine for thrombosis: A precise diagnosis and treatment strategy. Nano-Micro Lett. 2020, 12, 96.
- Solaimuthu, A.; Vijayan, A.N.; Murali, P.; Korrapati, P.S. Nano-biosensors and their relevance in tissue engineering. Curr. Opin. Biomed. Eng. 2020, 13, 84–93.
- Lenz, T.; Nicol, P.; Castellanos, M.I.; Engel, L.-C.; Lahmann, A.L.; Alexiou, C.; Joner, M. Small dimension—big impact! Nanoparticle-enhanced non-invasive and intravascular molecular imaging of atherosclerosis in vivo. Molecules 2020, 25, 1029.
- Christodoulides, N.; McRae, M.P.; Simmons, G.W.; Modak, S.S.; McDevitt, J.T. Sensors that learn: The evolution from taste fingerprints to patterns of early disease detection. Micromachines 2019, 10, 251.
- Li, T.; Feng, Z.-Q.; Qu, M.; Yan, K.; Yuan, T.; Gao, B.; Wang, T.; Dong, W.; Zheng, J. Core/shell piezoelectric nanofibers with spatial self-orientated β-phase nanocrystals for real-time micropressure monitoring of cardiovascular walls. ACS Nano 2019, 13, 10062–10073.
- Jiang, W.; Rutherford, D.; Vuong, T.; Liu, H. Nanomaterials for treating cardiovascular diseases: A review. Bioact. Mater. 2017, 2, 185–198.
- Barani, M.; Bilal, M.; Rahdar, A.; Arshad, R.; Kumar, A.; Hamishekar, H.; Kyzas, G.Z. Nanodiagnosis and nanotreatment of colorectal cancer: An overview. J. Nanopart. Res. 2021, 23, 18.
- Barani, M.; Bilal, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanotechnology in ovarian cancer: Diagnosis and treatment. Life Sci. 2020, 266, 118914.
- Barani, M.; Mukhtar, M.; Rahdar, A.; Sargazi, G.; Thysiadou, A.; Kyzas, G.Z. Progress in the application of nanoparticles and graphene as drug carriers and on the diagnosis of brain infections. Molecules 2021, 26, 186.
- Barani, M.; Mukhtar, M.; Rahdar, A.; Sargazi, S.; Pandey, S.; Kang, M. Recent advances in nanotechnology-based diagnosis and treatments of human osteosarcoma. Biosensors 2021, 11, 55.
- Ghazy, E.; Kumar, A.; Barani, M.; Kaur, I.; Rahdar, A.; Behl, T. Scrutinizing the therapeutic and diagnostic potential of nanotechnology in thyroid cancer: Edifying drug targeting by nano-oncotherapeutics. J. Drug Deliv. Sci. Technol. 2020, 61, 102221.
- Hasanein, P.; Rahdar, A.; Barani, M.; Baino, F.; Yari, S. Oil-in-water microemulsion encapsulation of antagonist drugs prevents renal ischemia-reperfusion injury in rats. Appl. Sci. 2021, 11, 1264.
- Mukhtar, M.; Bilal, M.; Rahdar, A.; Barani, M.; Arshad, R.; Behl, T.; Brisc, C.; Banica, F.; Bungau, S. Nanomaterials for diagnosis and treatment of brain cancer: Recent updates. Chemosensors 2020, 8, 117.
- Qindeel, M.; Barani, M.; Rahdar, A.; Arshad, R.; Cucchiarini, M. Nanomaterials for the diagnosis and treatment of urinary tract infections. Nanomaterials 2021, 11, 546.
- Rahdar, A.; Hajinezhad, M.R.; Sargazi, S.; Bilal, M.; Barani, M.; Karimi, P.; Kyzas, G.Z. Biochemical effects of deferasirox and deferasirox-loaded nanomicellesin iron-intoxicated rats. Life Sci. 2021, 270, 119146.
- Rahdar, A.; Sargazi, S.; Barani, M.; Shahraki, S.; Sabir, F.; Aboudzadeh, M.A. Lignin-stabilized doxorubicin microemulsions: Synthesis, physical characterization, and in vitro assessments. Polymers 2021, 13, 641.
- Sabir, F.; Barani, M.; Rahdar, A.; Bilal, M.; Nadeem, M. How to face skin cancer with nanomaterials: A review. Biointerface Res. Appl. Chem. 2021, 11, 11931–11955.
- Pala, R.; Pattnaik, S.; Busi, S.; Nauli, S.M. Nanomaterials as novel cardiovascular theranostics. Pharmaceutics 2021, 13, 348.
- Nicolosi, D.; Scalia, M.; Nicolosi, V.M.; Pignatello, R. Encapsulation in fusogenic liposomes broadens the spectrum of action of vancomycin against Gram-negative bacteria. Int. J. Antimicrob. Agents 2010, 35, 553–558.
- Darwitan, A.; Wong, Y.S.; Nguyen, L.T.; Czarny, B.; Vincent, A.; Nedumaran, A.M.; Tan, Y.F.; Muktabar, A.; Tang, J.K.; Ng, K.W. Liposomal nanotherapy for treatment of atherosclerosis. Adv. Healthc. Mater. 2020, 9, 2000465.
- Matoba, T.; Koga, J.-I.; Nakano, K.; Egashira, K.; Tsutsui, H. Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J. Cardiol. 2017, 70, 206–211.
- Dasa, S.S.K.; Suzuki, R.; Gutknecht, M.; Brinton, L.T.; Tian, Y.; Michaelsson, E.; Lindfors, L.; Klibanov, A.L.; French, B.A.; Kelly, K.A. Development of target-specific liposomes for delivering small molecule drugs after reperfused myocardial infarction. J. Control. Release 2015, 220, 556–567.
- Lestini, B.J.; Sagnella, S.M.; Xu, Z.; Shive, M.S.; Richter, N.J.; Jayaseharan, J.; Case, A.J.; Kottke-Marchant, K.; Anderson, J.M.; Marchant, R.E. Surface modification of liposomes for selective cell targeting in cardiovascular drug delivery. J. Control. Release 2002, 78, 235–247.
- Van der Valk, F.M.; van Wijk, D.F.; Lobatto, M.E.; Verberne, H.J.; Storm, G.; Willems, M.C.; Legemate, D.A.; Nederveen, A.J.; Calcagno, C.; Mani, V. Prednisolone-containing liposomes accumulate in human atherosclerotic macrophages upon intravenous administration. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1039–1046.
- Laing, S.T.; Moody, M.R.; Kim, H.; Smulevitz, B.; Huang, S.-L.; Holland, C.K.; McPherson, D.D.; Klegerman, M.E. Thrombolytic efficacy of tissue plasminogen activator-loaded echogenic liposomes in a rabbit thrombus model. Thromb. Res. 2012, 130, 629–635.
- Zhong, H.; Deng, Y.; Wang, X.; Yang, B. Multivesicular liposome formulation for the sustained delivery of breviscapine. Int. J. Pharm. 2005, 301, 15–24.
- Huang, G.; Zhou, Z.; Srinivasan, R.; Penn, M.S.; Kottke-Marchant, K.; Marchant, R.E.; Gupta, A.S. Affinity manipulation of surface-conjugated RGD peptide to modulate binding of liposomes to activated platelets. Biomaterials 2008, 29, 1676–1685.
- Januzzi, J.L., Jr.; Maisel, A.S.; Silver, M.; Xue, Y.; DeFilippi, C. Natriuretic peptide testing for predicting adverse events following heart failure hospitalization. Congest. Heart Fail. 2012, 18, S9–S13.
- Al Meslmani, B.M.; Mahmoud, G.F.; Bakowsky, U. Development of expanded polytetrafluoroethylene cardiovascular graft platform based on immobilization of poly lactic-co-glycolic acid nanoparticles using a wet chemical modification technique. Int. J. Pharm. 2017, 529, 238–244.
- Ahadian, S.; Huyer, L.D.; Estili, M.; Yee, B.; Smith, N.; Xu, Z.; Sun, Y.; Radisic, M. Moldable elastomeric polyester-carbon nanotube scaffolds for cardiac tissue engineering. Acta Biomater. 2017, 52, 81–91.
- Wennink, J.W.; Liu, Y.; Mäkinen, P.I.; Setaro, F.; de la Escosura, A.; Bourajjaj, M.; Lappalainen, J.P.; Holappa, L.P.; van den Dikkenberg, J.B.; Al Fartousi, M. Macrophage selective photodynamic therapy by meta-tetra (hydroxyphenyl) chlorin loaded polymeric micelles: A possible treatment for cardiovascular diseases. Eur. J. Pharm. Sci. 2017, 107, 112–125.
- Cormode, D.P.; Naha, P.C.; Fayad, Z.A. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast Media Mol. Imaging 2014, 9, 37–52.
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969.
- Nakashiro, S.; Matoba, T.; Umezu, R.; Koga, J.-i.; Tokutome, M.; Katsuki, S.; Nakano, K.; Sunagawa, K.; Egashira, K. Pioglitazone-incorporated nanoparticles prevent plaque destabilization and rupture by regulating monocyte/macrophage differentiation in ApoE−/− mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 491–500.
- Yoo, S.P.; Pineda, F.; Barrett, J.C.; Poon, C.; Tirrell, M.; Chung, E.J. Gadolinium-functionalized peptide amphiphile micelles for multimodal imaging of atherosclerotic lesions. ACS Omega 2016, 1, 996–1003.
- Kirana, C.; Rogers, P.F.; Bennett, L.E.; Abeywardena, M.Y.; Patten, G.S. Naturally derived micelles for rapid in vitro screening of potential cholesterol-lowering bioactives. J. Agric. Food Chem. 2005, 53, 4623–4627.
- Wang, S.; Wang, S.; Yu, X.; Zeng, J.; Li, H.; Yang, R.; Chen, W.; Dong, J. Magnetic nanoparticles functionalized with immobilized apolipoprotein antibodies for direct detection of non-high density lipoprotein cholesterol in human serum. Chem. Eng. J. 2020, 385, 123465.
- A Sharma, P.; Maheshwari, R.; Tekade, M.; Kumar Tekade, R. Nanomaterial based approaches for the diagnosis and therapy of cardiovascular diseases. Curr. Pharm. Des. 2015, 21, 4465–4478.
- Kievit, F.M.; Veiseh, O.; Fang, C.; Bhattarai, N.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano 2010, 4, 4587–4594.
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11.
- Mouli, S.K.; Tyler, P.; McDevitt, J.L.; Eifler, A.C.; Guo, Y.; Nicolai, J.; Lewandowski, R.J.; Li, W.; Procissi, D.; Ryu, R.K. Image-guided local delivery strategies enhance therapeutic nanoparticle uptake in solid tumors. ACS Nano 2013, 7, 7724–7733.
- Chen, C.-C.; Lin, Y.-P.; Wang, C.-W.; Tzeng, H.-C.; Wu, C.-H.; Chen, Y.-C.; Chen, C.-P.; Chen, L.-C.; Wu, Y.-C. DNA− gold nanorod conjugates for remote control of localized gene expression by near infrared irradiation. J. Am. Chem. Soc. 2006, 128, 3709–3715.
- Chithrani, B.D.; Chan, W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550.
- Ghann, W.E.; Aras, O.; Fleiter, T.; Daniel, M.-C. Syntheses and characterization of lisinopril-coated gold nanoparticles as highly stable targeted CT contrast agents in cardiovascular diseases. Langmuir 2012, 28, 10398–10408.
- Almutairi, A.; Rossin, R.; Shokeen, M.; Hagooly, A.; Ananth, A.; Capoccia, B.; Guillaudeu, S.; Abendschein, D.; Anderson, C.J.; Welch, M.J. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 685–690.
- Lu, Y.; Sun, B.; Li, C.; Schoenfisch, M.H. Structurally diverse nitric oxide-releasing poly(propylene imine) dendrimers. Chem. Mater. 2011, 23, 4227–4233.
- Tomalia, D.A. Starburstr̀ dendrimers—Nanoscopic supermolecules according to dendritic rules and principles. In Macromolecular Symposia; Hüthig & Wepf Verlag: Basel, Switzerland; pp. 243–255.
- Venkatesh, S.; Li, M.; Saito, T.; Tong, M.; Rashed, E.; Mareedu, S.; Zhai, P.; Bárcena, C.; López-Otín, C.; Yehia, G.; et al. Mitochondrial LonP1 protects cardiomyocytes from ischemia/reperfusion injury in vivo. J. Mol. Cell. Cardiol. 2019, 128, 38–50.
- Taite, L.J.; West, J.L. Poly (ethylene glycol)-lysine dendrimers for targeted delivery of nitric oxide. J. Biomater. Sci. Polym. Ed. 2006, 17, 1159–1172.
- Bhadra, D.; Bhadra, S.; Jain, N.K. Pegylated lysine based copolymeric dendritic micelles for solubilization and delivery of artemether. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Des Sci. Pharm. 2005, 8, 467–482.
- Shao, W.; Arghya, P.; Yiyong, M.; Rodes, L.; Prakash, S. Carbon nanotubes for use in medicine: Potentials and limitations. Synth. Appl. Carbon Nanotub. Compos. 2013, 13, 285–311.
- Chen, Z.; Zhang, X.; Yang, R.; Zhu, Z.; Chen, Y.; Tan, W. Single-walled carbon nanotubes as optical materials for biosensing. Nanoscale 2011, 3, 1949–1956.
- Strus, M.C.; Chiaramonti, A.N.; Kim, Y.L.; Jung, Y.J.; Keller, R.R. Accelerated reliability testing of highly aligned single-walled carbon nanotube networks subjected to DC electrical stressing. Nanotechnology 2011, 22, 265713.
- Garibaldi, S.; Brunelli, C.; Bavastrello, V.; Ghigliotti, G.; Nicolini, C. Carbon nanotube biocompatibility with cardiac muscle cells. Nanotechnology 2005, 17, 391.
- Jayagopal, A.; Russ, P.K.; Haselton, F.R. Surface engineering of quantum dots for in vivo vascular imaging. Bioconjugate Chem. 2007, 18, 1424–1433.
- Larson, D.R.; Zipfel, W.R.; Williams, R.M.; Clark, S.W.; Bruchez, M.P.; Wise, F.W.; Webb, W.W. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 2003, 300, 1434–1436.
- Ferrara, D.E.; Glaus, C.; Taylor, W.R. Targeting vascular epitopes using quantum dots. In Nanoparticles in Biomedical Imaging; Springer: Berlin/Heidelberg, Germany, 2008; pp. 443–461.
- Yan, M.; Zhang, Y.; Xu, K.; Fu, T.; Qin, H.; Zheng, X. An in vitro study of vascular endothelial toxicity of CdTe quantum dots. Toxicology 2011, 282, 94–103.
- Aizik, G.; Waiskopf, N.; Agbaria, M.; Ben-David-Naim, M.; Levi-Kalisman, Y.; Shahar, A.; Banin, U.; Golomb, G. Liposomes of quantum dots configured for passive and active delivery to tumor tissue. Nano Lett. 2019, 19, 5844–5852.
