Polysaccharides are polymeric carbohydrates composed of repeating monomeric units of monosaccharides that are covalently linked to each other through glucosidic linkage. Polysaccharides are biocompatible, biodegradable biopolymers, and the presence of various functional groups, such as hydroxyl, carboxyl, and amino groups, allow their easy chemical modification in order to increase their intrinsic properties (solubility, chemical stability, etc.). Due to these improved properties, polysaccharides are largely used as biomaterials in food, biomedical, pharmaceutical, cosmetic industry, and also as micellar drug-loaded systems.
- micelle
- self-assembly
- polysaccharides
1. Drug Delivery Systems Based on Cationic Polysaccharides
Chitosan (CS), the only naturally occurring cationic polymer, is renewable, inexpensive, and an environmentally friendly source of polycationic macromolecular building blocks owing to the chemical modification at the free amino (N-grafting) or hydroxyl (O-grafting) groups. The chemical structure of CS is provided in Figure 1:
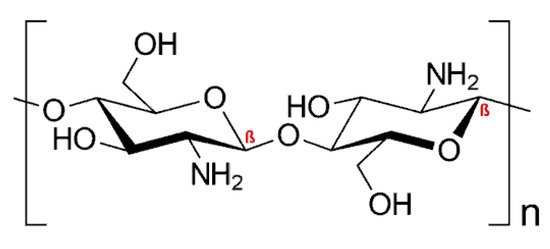
Figure 1. Chemical structure of chitosan (CS).
CS was modified with succinic anhydride and octaldelhyde followed by the conjugation of folate, as a ligand, in order to obtain micellar system for the tumor active targeting [1]. Relatively stable micelles for 15 days in phosphate-buffered saline (PBS) (pH = 7.4) and having an average diameter of 200 nm were prepared by a precipitation method followed by dialysis. Micellar DDS were obtained using fluorescein and indocyanine green (ICG) derivatives as hydrophobic model molecules. Both DEE and DLE were higher for fluorescein than for ICG derivatives due to the higher solubility. In vivo tests have clearly demonstrated the enhanced tumor targeting effect of these folate-functionalized CS-based micelles. Folate was also used as a ligand, in a more recent study, for the preparation, by precipitation followed by dialysis, of N-naphtlyl-N,O-succinyl CS micelles loaded with a semi-synthetic andrographolide analogue 3A.1 (19-tert-butyldiphenylsilyl-8,17-epoxy andrographolide) [2]. The size of these drug-loaded micelles was smaller than 200 nm and a negative ZP value of −31.7 mV was determined, indicating a good stability. As previously indicated, the folate moiety induced a higher anticancer activity against HT29 cancer cells than the non-functionalized micelles.
Stearic acid (SA) was used for the preparation of a series of amphiphilic copolymers based on low molecular weight CS, which self-assembled in aqueous medium in micelles with average sizes in the range of 33.4 to 130.9 nm and ZP values of 22.9 to 48.4 mV [3]. A SA substitution degree (DS) of around 40% led to smallest micellar size, C.M.C. value, and highest ZP value. Doxorubicin (Dox) was used as a model drug for in vitro and in vivo tests of these drug-loaded micellar systems, prepared by dialysis, as oral DDS, and it appeared that CS-based PMs increased the bioavailability of drug by prolonging its circulation time. The same hydrophobic modification was also performed by Moazeni et al. [4], but in this case, the micelles were loaded with itraconazale as a model drug for pulmonary delivery. The drug-loaded micelles, prepared by a thin film hydration method, had sizes in the range of 120 to 200 nm, and both DEE and DLE values were low. Moreover, the ZP values ranged from 34 to 74 mV, which were much higher than those obtained by Yuan et al. [3]. The nebulization efficiency was up to 89%, and these PMs maintained the drug stability during the inhalation process.
Fattahi et al. [5] have used CS-graft-retinoic acid micelles as gene carriers. Size, zeta, and C.M.C. of these micelles were 142.14 ± 5.06 mg/mL, 27.25 ± 6.31 mg/mL, and 1.3 × 10−2 mg/mL, respectively. The cellular uptake of these micelles was more effective in Hela than in HepG2 cells. The obtained results confirmed the fact that these micellar systems are safe and effective nanocarriers for in vitro and potentially in vivo gene delivery.
Xu et al. [6] have conjugated prostate cancer-binding peptides (PCP) with hydrophobic cholesterol-modified glycol CS (CHGC) at two different DS. Dox-loaded micelles were prepared by an emulsion/evaporation method, and it was noticed that the drug loading led to an increase of the micellar size from 246 to 293 nm. Moreover, DLE and DEE values were 11.4% and 85.8%, respectively. In vitro cytotoxicity and cellular uptake showed an enhanced intracellular drug delivery and a higher tumor inhibition owing to the presence of PCP. In another study, Muddineti et al. [7] have also used cholesterol in order to obtain an amphiphilic graft copolymer based on CS. The dialysis method was used for the preparation of Cur-loaded micelles, and the average values for DEE and DLE were 35.9% and 35.0%, respectively. The micelles prepared from the optimum formulation, having CS and cholesterol amounts of 10 mg and 5.24 mg, had an average diameter of 162 nm. In vitro cytotoxicity tests were performed on both murine melanoma and human breast cancer cells and showed an enhanced cytotoxic effect compared to free Cur. More recently, the same group has loaded both Cur and siRNA in the same type of PMs [8]. In this case, the average micellar diameter was 165 nm, and a ZP value of 24.8 mV was determined. Another amphiphilic graft copolymer was synthesized by the modification of CS with hydrophobic oleic acid (OA) [9]. Micelles, with an average diameter of 520 nm and a ZP value of 42 mV, were obtained by a thin film hydration method and were loaded with cefixime trihydrate (CFX). The influence of pH on drug-loading efficiency was investigated, and it appeared that DLE was maximum (55%) at pH = 5.5. The in vitro release studies revealed 52% of drug release after 24 h of incubation and were enhanced to 83% after 72 h in simulated intestinal fluid condition.
Recently, gambogic acid was loaded in O,N-hydroxyethyl CS-graft-octylamine graft copolymer micelles [10]. The coating with hyaluronic acid (HA) of these micelles, obtained by a dialysis method, has not affected the micellar size or the loading capacity. However, a ZP inversion, from around 20 to −24 mV, was observed after the HA coating. The efficiency of these micellar systems as DDS was investigated by in vitro and in vivo tests, and it was demonstrated that the HA-coated drug-loaded micelles have a higher antitumoral efficiency than the non-coated ones.
Hydrophilic mPEG was used for the synthesis of an amphiphilic CS-graft-mPEG graft copolymer, with a DS of 3.23% [11]. Starting from this copolymer, spherical micelles, with an average diameter of 200 nm, were prepared by an ultrasonication method. The loading of 5-fluorouracil (5-FU), as a model antitumoral drug, led to an average diameter of 769 nm for a 5-FU/CS-graft-mPEG weight ratio of 5/10 (w/w). Zhao et al. [12] prepared a similar micellar DDS which was further loaded with Dox by a dialysis method. These drug-loaded micelles, with a core of 64 nm and a corona of 14 nm in PBS at pH = 7.4, were pH-sensitive due to the ability of CS to switch from hydrophilic to hydrophobic as the pH switched from pH < 5 to pH = 7.4.
An interesting dual-targeting micellar system, based on succinyl-CS, folic acid (FA), and methianine (Met), was prepared by Chen et al. [13]. In addition, a fluorescence probe was conjugated as a hydrophobic group. Paclitaxel (PTX) was loaded in these spherical micelles with an average diameter of 200 nm. It was demonstrated that the active targeting ability of these micellar DDS was enhanced by the conjugations of both FA and Met as ligands. PTX was also loaded in micelles prepared, by dialysis, from similar FA–cholesterol–CS biopolymer [14]. The same drug, PTX, was used for the preparation of drug-loaded micelles based on an amphiphilic CS-graft-α-tocophenol succinate copolymer [15]. Spherical drug-loaded micelles were obtained by an ultrasonication method, and the loading process increased the average micellar diameter from 35 to 142 nm. Moreover, these PTX-loaded micelles proved in vitro and in vivo increased antitumoral activity. PTX-loaded micelles were also prepared from an amphiphilic copolymer, based on CS, after the previous hydrophobic modification of CS with two hydrophobic moieties, deoxycholic acid (DA) and FA at DS values of 15.8% and 8%, respectively [16]. The synthesis route of the amphiphilic graft copolymer based on CS is illustrated in Figure 2.
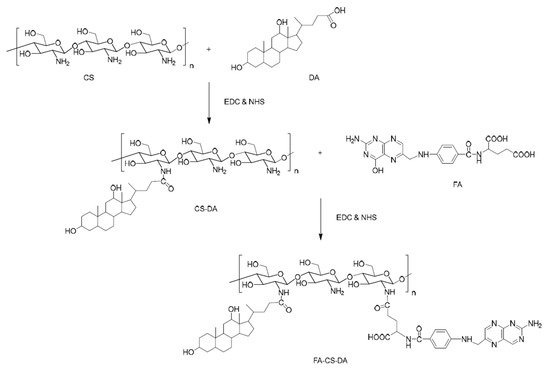
Figure 2. Schematic synthesis route of folic acid (FA)–CS–deoxycholic acid (DA) copolymer [16].
Spherical micelles, obtained by an ultrasonication method, were characterized by an average diameter of 126 nm and a positive ZP value of 19.3 mV. Moreover, the DLE and the DEE values of these PTX-loaded micelles were 9.1% and 81.2%, respectively. By performing in vitro and in vivo tests, it was concluded that these micellar systems are suitable carriers for the intravenous administration of PTX. More recently, a novel amphiphilic graft copolymer based on CS and N-octyl-N′-phthalyl-O-phosphoryl was synthesized and used for the loading and oral delivery of PTX [17]. The micellar system, with a mean diameter of 137 nm and a ZP value of 27.99 mV, was obtained by a dialysis method. In order to improve the stability in gastric fluids, this micellar system was filled into enteric-coated capsules. It have been demonstrated that this formulation increased the oral bioavailability of PTX for transcytosis across entorecytes. Another very recent study concerning the improvement of the oral bioavailability of PTX was carried out by Yang et al. [18]. The micellar DDS is based on L-carnitine (LC) conjugated CS–SA polymeric micelles, which were prepared by solvent evaporation–hydration method. The ligand LC was conjugated onto the micelle surface by anchoring its derivative stearoyl group to the lipophilic SA micellar core. The PTX-loaded micelles showed regular spherical shapes with relatively small average diameters of 157.1 nm and high DLE of 15.96%. Moreover, the micellar stability in water was supported by a low C.M.C. value of 14.31 ± 0.21 μg/mL. Furthermore, these drug-loaded micelles presented a slow and incomplete in vitro PTX release, and the pharmacokinetic studies indicated an increase of the relative bioavailability of PTX to 165.8% against Taxol, which is a commercial formulation. In another very recent study, a comparison between the efficiency of the commercial formulation Taxol and that of a redox-sensitive CS derivative modified with Ch, sulfhydryl, and methoxy-PEG (mPEG) (mPEG-CS(SH)-CHO) was also investigated [19]. The optimized PTX-loaded micelles, prepared by an ultrasonication method, had an average micellar diameter of 158 nm, ZP of +26.9 mV, DLE of 11.7%, and DEE of 88.3%. The PTX-loaded micelles possessed excellent in vivo anticancer effect compared to both control group and Taxol formulation, as illustrated in Figure 3.
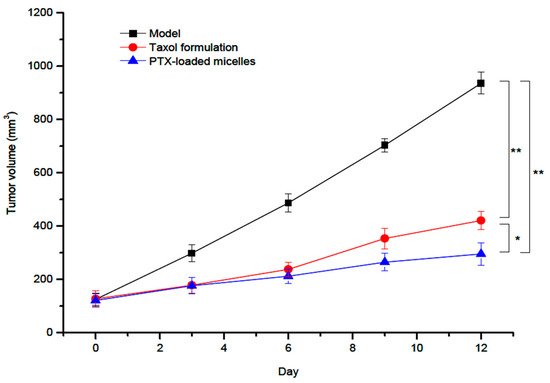
Figure 3. In vivo antitumor activity of Paclitaxel (PTX)-loaded mPEG-CS(SH)-CHO micelles: tumor volume growth curves after various treatments (* p < 0.05; ** p < 0.01) [19].
The PTX-loaded micelles exhibited a superior tumor inhibitory effect than Taxol, with a tumor inhibition rate of 67.3% vs. 53.8%.
To the best of our knowledge, no studies were conducted using oligochitosan for the synthesis of amphiphilic block copolymers, which are much easier to characterize than graft copolymers, for the further use as micellar DDS.
2. Drug Delivery Systems Based on Anionic Polysaccharides
Hyaluronic acid or hyaluronate (HA), a natural linear anionic biopolymer composed of d-glucuronic acid and N-acetyl-d-glucosamine alternating units (Figure 4), is biodegradable, biocompatible, nontoxic, non-thrombogenic, non-immunogenic, and non-inflammatory. Owing to these extraordinary properties, as well as to its easy chemical modification, it was possible to prepare various HA-based biomaterials with promising biomedical applications [20].
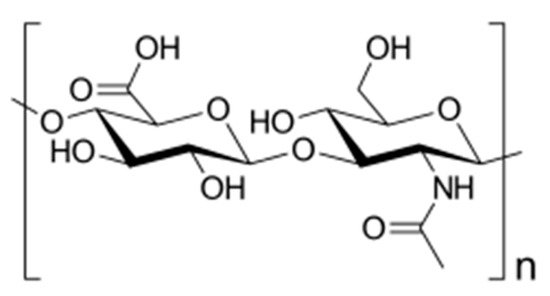
Figure 4. Chemical structure of hyaluronic acid (HA).
HA–SS-deoxycholic acid amphiphilic copolymer micelles were prepared and used for the loading, by dialysis, and release of PTX, as an antitumoral model drug [21]. The increase of the DS from 3% to 10% led to a decrease of both the C.M.C. values, from 161.3 to 34.7 mg/L, and average micellar diameters, from 292.4 to 128.1 nm. On the contrary, ZP values increased from −31 to −36.6 mV. Maximum DLE and DEE values were 34.1% and 93.2%, respectively and the drug-loading step induced a decrease of the micellar sizes and an increase of the ZP values. In the absence of glutathione (GSH), a release of 14.3% within 24 h was noticed, whereas in the presence of 10 mM GSH, the cumulative release was 52.1% and increased up to 90% at a concentration of 20 mM GSH due to the redox sensitivity of disulfide bonds. In another study, PTX was loaded in micelles of hydrophobically modified HA with C6 or C18 acyl chains copolymer [22]. Compared to the previous study, lower maximum DLE (14%) and DEE (70%) values were calculated. The average diameters of the spherical PTX-loaded micelles, prepared by a solvent evaporation method, ranged from 57 to 66 nm. Very recently, PTX was also loaded in mPEG–SS–HA–C16 graft copolymers micelles [23]. The optimal characteristics of these spherical micelles, obtained by sonication, were 168.6 nm, −38.3 mV, and a C.M.C. value of 1.05 × 10−2 mg/mL. Moreover, the maximum values of DLE and DEE were 14.7% and 86%, respectively. The drug release was studied in the absence and in the presence of 10 mM GSH, and it appeared that in the absence of GSH, a slow drug release (10.8%) was observed within 24 h, whereas in the presence of GSH, the cumulative release reached 74.3% within 24 h.
Cholesterol (Ch) was another hydrophobic molecule used for the modification of HA [24]. The obtained micelles, with average diameters smaller than 100 nm were loaded with Dox, superparamagnetic iron oxide nanoparticles (SPION), and magnetic resonance imaging (MRI) contrast agents by a dialysis method. The release of Dox was studied in the absence and in the presence of hyaluramidase enzyme, which degrades the HA and enhances the drug release. Moreover, higher micellar uptake was observed in cancer cells versus normal cells. In another study, hydrophobic Ch was loaded in micelles based on hydrophobically modified HA with 1,2-dimiristoyl-sn-glycerol-3-phosphatidylethanolamine (DMPE) or 1,2-distearoyl-sn-glycerol-3-phosphatidylethanolamine (DSPE) [25]. HA-DMPE micelles were characterized by a C.M.C. value of 16.5 μg/mL, an average diameter of 165 nm, and a ZP value of −21.9 mV, whereas the HA-DSPE had a C.M.C. value of 13.4 μg/mL, an average size of 214 nm, and a ZP value of −29.1 mV. An increase of the average diameters as well as a decrease of the ZP values was noticed after the drug loading.
A series of HA–graft–octodecylamine graft copolymers, with DS values ranged from 12% to 51%, were synthesized for the preparation of Dox-loaded micelles [26]. Increasing the DS led to a decrease of both the average diameters, from 221 to 109 nm, and ZP values, from −23 to −10 mV. DEE and DLE, which were also affected by the DS variation, have maximum values of 95.3% and 16%, respectively. The copolymer micelles obtained at a DS of 12 had the highest release rate, whereas the slowest was observed for a DS of 51. However, in vitro cytotoxicity and cellular uptake studies showed that micelles with a DS of 23 are characterized by the highest anticancer activity and internalization. In a more recent study, dodecylamine was grafted to HA, at different DS, in order to prepare pH-sensitive graft copolymer micelles loaded with Dox by dialysis and ultrasonic methods [27]. Increasing the DS from 17.3 to 28.4, the C.M.C. values decreased from 38.0 to 21.4 μg/mL, as expected. The increase of the DS led also to a slight increase of the DEE, from 68.9% to 84.3%, whereas DLE values remained almost constant in the range of 25% to 27%. Moreover, the loading of Dox increased slightly the micellar size (around 160 nm) and decreased the ZP values. Furthermore, the release of Dox was investigated as a function of pH, and it appeared that the release rate was higher at pH = 5.0 compared to pH = 7.4. The authors showed that these micelles exhibited great antitumor efficiency with low in vivo systemic toxicity. A dual encapsulation of Dox and Cur was carried out in micelles based on HA, vitamin E succinate (VES) and PEG2000 (TPGS2K) derivatives [28]. HA-graft-TPGS2K micelles were characterized by smaller average micellar diameter (153 nm) and ZP value (−10.4 mV) than HA-graft-VES micelles, which had an average size of 223 nm and a ZP value of −10.4 mV. However, no significant difference was noticed between these two types of micelles concerning the DLE values (around 7%). The release of Dox and Cur was pH-dependent with a fast release at pH = 4.5 and pH = 5.5 but a slow rate at pH = 6.5 and pH = 7.4. Moreover, an improved in vivo antitumoral effect was observed, however, without a clear difference between the studied micellar systems.
Docetaxel (DTX) and SPION were co-encapsulated in hexadocylamine-modified HA micelles for dual tumor targeted MR imaging and combined chemo-phototherapy [29]. Micelles were obtained by dialysis, and the drug-loading step led to a decrease of both the average micellar diameters, from 150 to 130 nm, and ZP values from −28 to around −17 mV. It appeared that the DTX release could be accelerated by external near infra-red (NIR) laser irradiation in order to have an on-demand drug release at the tumor site. DTX was also loaded in micelles based on reduction responsive core-crosslinked HA-block-poly(trimethylene carbonate-co-dithiolene trimethylene carbonate) (HA-CCMs) block copolymer [30]. The micelles, obtained by a precipitation method followed by dialysis, loaded with 6.6 wt % DTX displayed a narrow size of 85 nm. A further increase of the drug amount up to 20% led to an average diameter of 94 nm. The ZP values were in the range of −26 to −29 mV. The maximum DLE and DEE values were 40% and 9.1%, respectively. The cumulative release of DTX after 24 h in the absence of GSH was around 20%, whereas it was almost 100% in the presence of 10 mM GSH, indicating the rapid reduction–responsivity of micelles. These micellar systems demonstrated a long blood circulation time, strong tumor accumulation, and deep tumor penetration.
Bongiovi et al. [31] have prepared an interesting micellar system, based also on HA, hexadecyl chains (C16), ethylenediamine (EDA), PEG, and/or l-carnitine (CRN), which was loaded with imatinib, a small molecule kinase inhibitor of ABL1. The maximum DLE value of 13% was obtained for HA–EDA–C16 micelles, whereas lower values were noticed for HA–EDA–C16–PEG and HA–EDA–C16–CRN micelles. The average diameters of both blank and drug-loaded micelles was influenced by the dispersion medium, smallest values being generally observed in 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) medium. Figure 5 illustrates the variation of the average diameters of drug-loaded micelles as a function of the dispersion media.
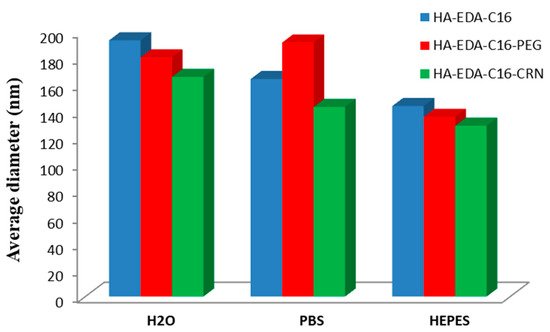
Figure 5. Variation of average micellar diameters of drug-loaded micelles in bidistilled water, PBS and 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffer (redrawn from [31]).
The potential advantage of these micellar systems is that they can be administered non-invasively, i.e., by topical ocular instillation, and it appeared that these imatinib-loaded micelles were able to interact with corneal barrier and promote imatinib transcorneal permeation and penetration.
Alginate, another naturally occurring anionic polysaccharide, is composed of two conformational isomer residues: β-d-mannuronic acid (M) and α-l-guluronic acid (G), as illustrated in Figure 6.
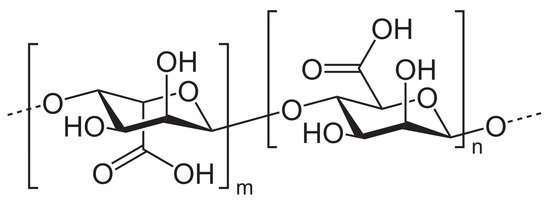
Figure 6. Chemical structure of alginic acid.
The potential applications of alginate biopolymers are restricted because of its low water solubility and high solution viscosity; therefore, their chemical modification is necessary [32].
Poly(N-isopropylacrylamide) (PNIPAM) sequences were grafted on alginate backbone in order to obtain thermo-sensitive alginate–graft–PNIPAM copolymer micelles which were loaded with Dox by dialysis at 37 °C [33]. At this temperature, PNIPAM sequences become hydrophobic and form the micellar core. Molecular weight of PNIPAM grafts, DS, and copolymer concentration have an important influence of the colloidal characteristics of these micelles. For example, the increase of the DS from 5 to 20 has as a consequence the decrease of the C.M.C. values from 0.04 to 0.12 mg/mL, as expected. Moreover, increasing the Mn of the PNIPAM sequence, from 1000 to 4000 g/mol, led to an increase of the average diameters from 400 to around 1000 nm. Dox was loaded into these micelles with a maximum DLE value of 62.7% and a DLL of 24.2%. A sustained release of Dox, more than 90%, was achieved within 96 h. Yu et al. [34] have obtained similar thermo-sensitive sodium alginate–graft–PNIPAM graft copolymer micelles for the loading and delivery of 5-FU. However, in this case, the core of the micelles was constituted by sodium alginate crosslinked with metal ions (Ba2+, Zn2+ and Co2+) whereas the thermo-sensitive PNIPAM formed the corona. As a function of the metal ion used as crosslinking agents, the average diameters ranged from 50 to 340 nm. Moreover, the ZP varied from −22 to −27 mV. Furthermore, maximum DLE and DEE values were obtained for micelles crosslinked with Ba2+. Increasing the temperature and ionic strength or decreasing pH, a fast 5-FU release was observed.
Dodecyl glycidyl ether (DGE) was used for the hydrophobic modification of alginate in order to prepare drug-loaded micelles [35]. Sudan IV was encapsulated in these micelles, as a hydrophobic model drug. Owing to their amphiphilic behavior, the surface tension was investigated and values in the range of 25 to 30 mN/m were determined at a C.M.C. value of 0.1 mg/mL. The average diameters of these spherical micelles increased from 1 to 5 μm with increasing the copolymer concentration, whereas an almost constant ZP value of −82.85 mV was noticed independent of the copolymer concentration. Another hydrophobically modified and pH-responsive amphiphilic alginate copolymer was synthesized via Ugi reaction and used for the preparation of drug-loaded micelles by direct dissolution [36]. The impact of Na+ concentration on the micellar sizes was studied, and it appeared that the decrease of the ionic strength led to an increase of the average micellar diameters. TEM images showed that the mean micellar sizes were between 30 and 200 nm. The loading of a model pesticide (acetamiprid) was also influenced by the ionic strength and pH.
3. Drug Delivery Systems Based on Non-Ionic Polysaccharides
Cellulose, composed of β-1,4-linked d-glucose units (Figure 7), is the most abundant biopolymer and has very interesting properties, such as biodegradability, renewability, high strength, and high modulus, but its practical applicability is limited by extremely poor solubility in water and common organic solvents. In order to be used as micellar DDS, cellulose has to be modified for the preparation of amphiphilic copolymers.
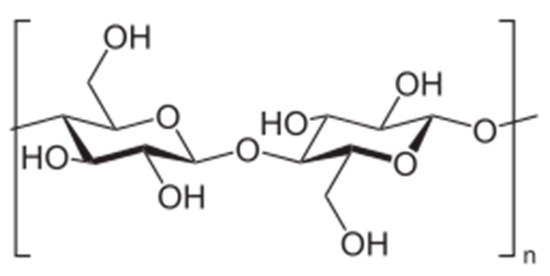
Figure 7. Chemical structure of cellulose.
Amphiphilic cationic cellulose (HMQC) copolymers composed of long-chain alkyl groups, as hydrophobic sequences, and quaternary ammonium groups, as hydrophilic moieties, were used for the preparation of preduisone acetate-loaded micelles with average radii in the range of 320 to 430 nm [37]. The drug-loading step decreased the micellar sizes but increased slightly the ZP values, which ranged from 56.5 to 61.9 mV. The spherical morphology of these micelles, prepared by a dialysis method, was observed by TEM. Both DLE and DEE values were in the range of 16% to 22%, and a cumulative drug release of 86.5% was observed after 120 h, indicating a controlled and sustained drug release. In another study, cellulose was modified with 4-(N-methylamino) butyrate hydrochlorides for the preparation of camptothecin (CPT)-loaded micelles [38]. The micelles were prepared by a dialysis method, and their spherical morphology was assessed by SEM. As previously reported, the drug encapsulation process, with an encapsulation efficiency of about 86.4%, led to a decrease of the average micellar diameters from 495 to 233 nm due to stronger hydrophobic interactions between the drug and the hydrophobic micellar core. The drug release tests indicated a sustained release of CPT to >80% over 4 days at both pH = 6.8 and pH = 7.4.
Amphiphilic cellulose–graft–poly(p-dioxanone) (MCCC-graft-PPDO) copolymer micelles were prepared by the dialysis method in order to encapsulate hydrophobic fluorescent conjugated polymers (FCPs) for tumor cell imaging [39]. After the loading of FCPs, the average micellar diameters increased from 40 nm to about 100 ÷ 200 nm and were stable over time. Moreover, DLL values increased with increasing the FCPs concentration, whereas the DEE values decreased. Furthermore, it was demonstrated that the optimal DEE and DLL values could by obtained with 1000 μg/mL MCCC–graft–PPDO and 75 μg/mL FCPs, and that these FCPs-loaded micelles are successfully uptaken by the cancer cells and located at the cytoplasm of the cells, suggesting their great potential for tumor cell imaging and early detection of tumor cells.
Carboxymethyl cellulose–graft–polylactic acid (C.M.C-graft-PLA) copolymer was functionalized with anti-EpCAM antibody by an amidation reaction for the active delivery of both Dox and HepG2 cells [40]. These drug-loaded micelles, prepared by an emulsification method followed by dialysis, have average micellar diameters of around 150 nm and were stable for 7 days. Moreover, these micelles were suitable for intravenous injections, as the C.M.C. was low (0.043 mg/mL). Moreover, by increasing the amount of loaded drug, it appeared that DLL increased from 8.2% to 12.6%, whereas DEE decreases from 81.6% to 36%. As the pH value decreases, the solubility of Dox increases, and therefore, the drug release rate from these micelles will increase. Furthermore, the functionalized Dox-loaded micelles exhibited better antitumor and therapeutic effects than both free drug and non-functionalized drug-loaded micelles.
Dextran (DEX), composed predominantly of α-1,6-linked glucopyranose units with a low degree of 1,3-branching (Figure 8), is a highly water-soluble polysaccharide of bacterial origin, produced by Lactobacillus, Leuconostoc, and Streptococcus species. In order to prepare amphiphilic copolymers, DEX was hydrophobically modified with different synthetic polymers or other hydrophobic molecules.
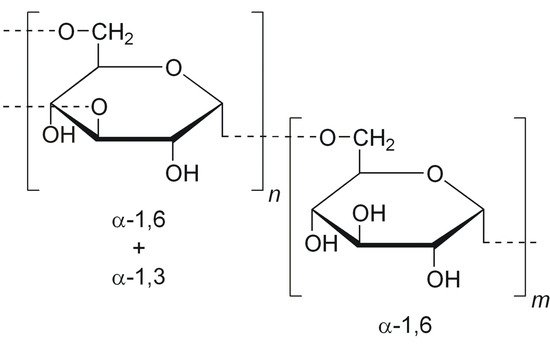
Figure 8. Chemical structure of dextran (DEX).
An interesting study was related to the synthesis of an amphiphilic DEX–SS–poly(ε-caprolactone) (PCL) block copolymer in which PCL was linked to DEX by a disulfide bond [41]. Dox was loaded, by a dialysis method, into micelles having a C.M.C. value of 9.3 mg/L. The drug-loading step increased the micellar average diameter from 60 to 80 nm. The DLE value was around 70%, and a minimal drug release (<20%) was noticed within 20 h. From the in vitro tests, it appeared that Dox loaded in these reduction–responsive biodegradable micelles is delivered and released to the cytoplasm as well as to the cell nucleus, achieving higher drug efficacy, due to the reduction–responsive SS bonds, than the drug loaded in reduction-insensitive Dex–PCL micelles. More recently, Dox was also loaded, by dialysis, into DEX–graft–PCL graft copolymer micelles [42]. The maximum values for DEE (42.2%) and DLE (17.4%) were obtained for a Dox concentration of 0.3 mg/mL. The cumulative release at pH = 5.2 was greater than that at pH = 7.4, which was probably due to the protonation of Dox in acidic environment. Furthermore, the cytotoxicity tests reflected the biocompatibility of the micelles. In another study, Dox was also loaded in DEX–graft–stearate amphiphilic graft copolymers micelles [43]. Depending on both the DS of SA and the molar mass (Mn) of DEX, the C.M.C. values ranged from 0.01 mg/mL to 0.08 mg/mL whereas the hydrodynamic diameter ranged from 24 to 258 nm. However, in this study, the drug loading has not affected the micellar size. DEE values reached up to 92.23%, while a maximum DLL value of 14.41% was determined. A prolonged in vitro Dox release, up to 48 h, was noticed as a function of the Mn of DEX, DS of SA, or the drug-loading content. Moreover, in vivo antitumor activity tests showed that the tumor growth was suppressed to a higher extent in the presence of these Dox-loaded micelles as compared with commercial Dox injection. Another group has prepared the same type of graft copolymer by the acylation of DEX with stearoyl chloride, at different DS, but in this study, etoposide was used as a model drug [44]. Compared to the previous study, an increase of the average diameters of these micelles was observed after the drug loading, whereas an opposite effect was noticed for the ZP values. For example, for the DEX6-SA7.5 sample, the average diameter increased from 131.7 to 169.6 nm, but the ZP decreased from −30 to −19.2 mV. Moreover, it appeared clearly that the DS has a direct influence on both the DEE and DLE, the maximum values being 31.7% and 93.3%, respectively. A maximum cumulative drug release of around 80% was observed after 48 h in PBS at pH = 7.4. In another study, DEX was further modified with stearyl acid, and the obtained micelles, with a C.M.C. value of 1.8 mg/L, were loaded by a precipitation method with both Dox and supermagnetic iron oxide (SPIO) nanocrystals [45]. These drug-loaded micelles were characterized by an average diameter of 100 nm and showed good biocompatibility and excellent internationalization ability against MCF-7/Adr cells. Dox and SPIO nanoparticles were also encapsulated in A54 peptide-functionalized DEX–graft–PLGA graft copolymer micelles with a C.M.C. value of 22.51 μg/mL [46]. The average micellar diameter increased from 50 to 100 nm after the drug loading with DEE and DLE values of 80% and 4%, respectively. A cumulative Dox release of around 80% was noticed after 72 h in PBS at both pH = 5.5 and pH = 7.2. Moreover, these peptide-functionalized Dox-loaded micelles showed better therapeutic effects than the non-functionalized micelles. Dox was also loaded, via a precipitation method, into DEX–block–PLA block copolymer micelles with average diameters in the range of 15 to 70 nm, as a function of the molecular characteristics of the copolymer [47]. Maximum DLE and DEE values of around 21.2% and 45%, respectively were determined. In vitro hemolysis results showed that these micellar systems are suitable as injectable DDS. More recently, other Dox-loaded DEX–block–PLA micelles, with average diameters ranged from 158 to 182 nm, were prepared by a dialysis method [48]. The C.M.C. values decreased from 6.63% to 3.87% as the Mn of the PLA sequence increased from 2000 to 4000 g/mol. Moreover, the maximum DLE and DEE values were 12.6% and 75%, respectively. A maximum cumulative drug release of around 60% was observed after 72 h in PBS at 37 °C and pH = 7.4. In a similar study, Dox was loaded into DL-lactide-co-glycolide-block-DEX spherical micelles by a dialysis method [49]. After the drug loading, the average micellar diameters increased from 82.6 to 206 nm for a drug content of 12.3%. In addition, it appeared that higher initial drug feeding induced higher drug content and slower release rate.
The same model drug, Dox, was loaded, by Yu et al. [50], with DEX-graft-α-tocopherol succinate graft copolymer micelles prepared by a dialysis method. Both micellar sizes and DLE were increased with increasing the drug/micelles ratio and this tendency is illustrated in Figure 8.
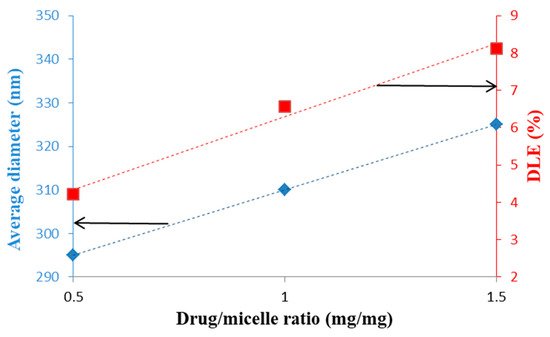
Figure 9. Evolution of the average micellar diameters and DLE as a function of the Dox/micelle ratio (mg/mg) (redrawn from [50]).
On the contrary, this increase of the Dox/micelle ratio has as consequence the decrease of DEE values from 87.9% to 58.9%. A spherical morphology was observed by TEM and a release percentage of 50.2% was determined after 96 h. This slow release was induced either by the hydrophobic/hydrophobic or van der Waals interactions between Dox and the hydrophobic sequences of the copolymer. In vitro cytotoxicity studies revealed that these drug-loaded micelles were practically non-toxic and therefore suitable as DDS.
Another type of DEX-based DDS was related to Cur-loaded DEX-graft-casein graft copolymers micelles with an average diameter of 75.3 nm and a ZP value of −18.1 mV [51]. The stability of these micelles was determined by turbidimetry in the pH range of 2 to 8, and it appeared that these PMs are more stable than the casein micelles. Another group has loaded Cur, by dialysis, in oxidized DEX–SA spherical micelles with average diameters smaller than 50 nm [52]. The C.M.C. values ranged from 0.07 to 0.158 mg/mL as a function of the DS of SA. As expected, the DLE values increased with increasing the initial drug feeding, whereas an opposite effect was noticed for DEE. Moreover, in vitro drug release was prolonged by adjusting the amount of loaded Cur. Cur was also loaded in other amphiphilic block copolymer micelles based on two types of DEX, such as hydrophilic and hydrophobic acetalated DEX. This double-based DEX copolymer self-assembles in water at a low C.M.C. value of 12 mg/L. The Cur loading, by dialysis, led to a slight decrease of the average micellar diameter from 103 to 98.9 nm. In addition, the drug loading decreased the ZP values from 2.8 to −6.1 mV. The degradability of this micellar system under acidic conditions was investigated, and it appeared that demicellization occurs after 2 h at pH = 5.5 due to the cleavage of acetal groups. On the contrary, it was shown that this micellar system is stable up to four months at a neutral pH value.
Blanco-Fernandez et al. [53] studied a thermo-sensitive micellar system based on DEX grafted with poly(isopropylacrylamide) (PNIPAM) and loaded with methotrexate (MTX) at two pH values, such as 5.5 and 7.4. Only unimers were observed at 20 °C, whereas at 37 °C, the C.M.C. value was 80 μg/mL. At 37 °C and a copolymer concentration of 1 mg/mL, the average micellar diameter was 190 nm. Moreover, the drug-loading efficiency was higher at pH = 7.4 than at pH = 5.5. As expected, MTX-loaded micelles showed pH- and thermo-responsive release and a higher cytotoxic effect than that of free MTX. Another pH-sensitive graft copolymer with controlled Mn and narrow dispersity, based on DEX and poly(oleic acid), has been synthesized by reversible addition-fragmentation chain transfer (RAFT) polymerization [54]. By dialysis, were obtained spherical micelles, from a C.M.C. value of 0.04 mg/mL, which were further loaded with nifedipine (NFD), as a model drug. Increasing the copolymer concentration from 0.03 to 0.05 mg/mL led to the increase of the average micellar diameters from 201.5 to 264.3 nm. Moreover, the drug loading increased slightly these diameters and the ZP value from −16.7 to −28 mV. Furthermore, DEE and DLE values were calculated to be 41.42% and 10.35%, respectively. The NFD release rate was faster at pH = 1.2 than at pH = 7.4.
Starch is composed of amylose, a linear polymer of glucose unit with α-(1→4) linkage, and amylopectin, which is highly branched with lots of short chains that linked through α-(1→6) linkage. In order to overcome the drawbacks of native starch, such as poor processability and solubility in common organic solvents, retrogradation and syneresis, low shear stress resistance and thermal decomposition, several types of modifications are possible: physical, chemical, enzymatic and genetic engineering, respectively [55].
The core of starch–graft–PEG2000 or 5000 micelles was crosslinked in the presence of lipoic acid via disulfide bonds in order to study the reduction–responsive behavior in the presence of GSH [56]. Dox was loaded, by a dialysis method, as a model drug, and the crosslinking of the micellar core increases both DLE and DEE values, the maximum values being equal to 9.52% and 57.12%, respectively. Moreover, this core crosslinking led to a decrease of the average micellar diameters from around 300 to approximatively 200 nm. In the presence of 10 mM GSH, these micelles exhibit faster drug release behavior and higher cellular proliferation inhibition efficiency. In another similar study, 3,3′-dithiodipropionic acid (DPA) was used as crosslinking agent in order to obtain Dox-loaded micelles, by a direct dissolution method, with a hydrophobic crosslinked starch core and a hydrophilic mPEG corona [57]. The crosslinking process induces the decrease of both average micellar diameters and C.M.C. values due to the formation of a more compact hydrophobic core. The micellar stability was investigated during 48 h in PBS at 37 °C, and it appeared that only a slight increase of the micellar sizes occurred during this period. However, an important increase of the micellar size was observed, due to the cleavage of the SS bonds and thus micellar aggregation, in 10 mM GSH solution. As previously indicated, this reduction–responsive behavior has an influence also on the Dox release rate.
Another amphiphilic glucose-sensitive dialdehyde starch polymer containing 3-aminophenylboronic acid (APBA) as a glucose-responsive group and mPEGylated dialdehyde starch (mPEG-DAS) with hydrophobic 7-hydroxycoumarin-4-acetic acid (Cou) was synthesized by Wen et al. and used for the preparation of insulin-loaded micelles by a sonication method [58]. The conjugation of Cou decreased the micellar size from 241.4 to 177.9 nm, and the C.M.C. values increased as the DS of Cou increased. Insulin was loaded into these micelles with a DLE of 30.4% and a DLL of 9.4%. A cumulative drug release of around 55% was observed within 50 h in the presence of 3 g/L glucose while demonstrating comparatively inert release at a glucose concentration of 1 mg/mL. A similar study was conducted by the same research team [59]. However, in this case, sulfobetaine formed the hydrophilic corona of the micelles.
Classical starch–graft–PEG graft copolymers were synthesized from tapioca starch biopolymer for the preparation of Cur-loaded micelles [60]. Empty spherical micelles, prepared by direct dissolution, had an average micellar diameter around 100 nm and a C.M.C. value of 0.0765 mg/mL, at pH = 7.4. A maximum DLE value of 5.6% and a DEE of 39.4% were obtained when 100 mg of polymer was added to 10 mg of Cur. After 95 h, cumulative Cur release has higher at pH = 5.5 (23%) than at pH = 7.4 (8%). In a similar study, PEG 5000 and TG100-115, which is an exclusive PI3Kc inhibitor, were conjugated on hydroxyethyl starch in order to obtain sorafenid-loaded micelles by a dialysis method [61]. The drug release was investigated as a function of pH (5.5, 6.8, and 7.4), and it appeared that the higher release rate was observed at low pH value. Moreover, a pharmacokinetic study showed that these micelles had longer half-life than the solution of the free drug, which was favorable for high propensity of extravasation through tumor vascular fenestrations. Furthermore, under low pH and high a-amylase reductive conditions, a quick demicellization occurs, thus enhancing significantly the cytotoxic activity against Hep-3B liver cancer cells. Finally, starch was modified with octenyl succinic anhydride in order to obtain β-carotene-loaded micelles [62]. As expected, the C.M.C. values depended on both the DS and Mw of the graft copolymer. Empty spherical micelles, with sizes smaller than 20 nm, were prepared by direct dissolution in hot water and the drug loading led to an important increase of the average micellar diameters up to 1000 nm.
4. Drug Delivery Systems Based on Miscellaneous Polysaccharides
Table 1 gives several examples of drug delivery systems based on less studied polysaccharides.
Table 1. Drug delivery systems based on miscellaneous polysaccharides.
| Copolymer | Drug | Size (nm) | ZP (mV) | Preparation Method | Reference |
|---|---|---|---|---|---|
| Xanthan–graft–C16 alkyl chain | Glibenclamide | 652.8 | −27.6 | Precipitation/evaporation | [63] |
| Pullulan–graft–PCL | ciprofloxacin | 142 | - | Dialysis | [64] |
| Pullulan–graft–α-tocopherol | CPT | 171.5 ÷ 257.7 | - | Dialysis | [65] |
| Pullulan–graft-SA | Dox | 188.75 | 17.83 | Dialysis | [66] |
| Pullulan–graft–retinoic acid+biotin | Dox | 191 | −9.45 | Direct dissolution | [67] |
| Pullulan–graft–desaxycholic acid–graft–PEI | Dox DNA |
160.8 | 28 | Dialysis | [68] |
| Pulullan–graft–retinoic acid | Dox | 124.5 | −3.65 | Direct dissolution | [69] |
| Pullulan–graft–(dibuthyl amino) propyl carbamate | DNA | 246.3 ÷ 1214 | 24.7 ÷ 47 | Dialysis | [70] |
| Pullulan–graft–cholesterol | methatrexate | 97.8 144.4 178.0 |
4.29 3.392.56 |
Dialysis | [71] |
| Heparin–graft–β-sitosterol cysteamine |
Dox | 93.3 | −41.8 | Sonication and dialysis |
[72] |
| Heparin–graft–α-tocopherol | Docetaxel | 139.7 | −24.6 | Dialysis | [73] |
| Chitin–graft–hexadecyl | Dox | 332.4 ÷ 385.0 | 38.2 ÷ 44.8 | Dialysis | [74] |
| Guar gum galactomannan–graft–acetic anhydride | Cur | 113.9 ÷ 152.8 | −14.8 ÷ −18.1 | Precipitation/solvent diffusion | [75] |
| Bletilla striata–graft–SA | Dox | 124.17 ÷ 145.93 | −13.17 ÷ −18.97 | Dialysis | [76] |
| Phthalated cashew gum | Benznidazole | 288.8 | −31.8 | Precipitation | [77] |
| Schizophyllan–graft–SA | PTX | 156 ÷ 175 | −28 ÷ 35.14 | Sonication | [78] |
| Fucoidan–graft–octenyl succinic anhydride | Cur | 103.9 | −43.57 | Not indicated | [79] |
| Poly(1-O-methacryloyl-β-d-fructopyranose)-block-poly(methyl methacylate) | PTX | 25 ÷ 46 | −9.9 ÷ −22.3 | Dialysis | [80] |
From Table 1, it appears that dialysis is generally the preferred method for the preparation of biopolymers-based micellar DDS.
This entry is adapted from the peer-reviewed paper 10.3390/polym13030477
References
- Zhu, H.; Liu, F.; Guo, J.; Xue, J.; Qian, Z.; Gu, Y. Folate-modified chitosan micelles with enhanced tumor targeting evaluated by near infrared imaging system. Carbohydr. Polym. 2011, 86, 1118–1129.
- Kansom, T.; Dumkliang, E.; Patrojanasophon, P.; Sajomsang, W.; Saeeng, R.; Zhu, W.M.; Opanasopit, P. Folate-Functionalized Amphiphilic Chitosan Polymeric Micelles Containing Andrographolide Analogue (3A.1) for Colorectal Cancer. Key Eng. Mater. 2019, 819, 15–20.
- Yuan, H.; Lu, L.-J.; Du, Y.-Z.; Hu, F.-Q. Stearic Acid-g-chitosan Polymeric Micelle for Oral Drug Delivery: In Vitro Transport and in Vivo Absorption. Mol. Pharm. 2010, 8, 225–238.
- Moazeni, E.; Gilani, K.; Najafabadi, A.R.; Rouini, M.; Mohajel, N.; Amini, M.; Barghi, M.A. Preparation and evaluation of inhalable itraconazole chitosan based polymeric micelles. DARU J. Pharm. Sci. 2012, 20, 85.
- Fattahi, A.; Golozar, M.A.; Varshosaz, J. Retinoic acid-oligomeric chitosan micelles as novel gene delivery carrier; in vitro transfection study. J. Rep. Pharm. Sci. 2013, 2, 125–130.
- Xu, J.; Yu, J.; Xu, X.; Wang, L.; Liu, Y.; Li, L.; Zhao, J.; He, M. Development, Characterization, and Evaluation of PSMA-Targeted Glycol Chitosan Micelles for Prostate Cancer Therapy. J. Nanomater. 2014, 2014, 462356.
- Muddineti, O.S.; Kumari, P.; Ray, E.; Ghosh, B.; Biswas, S. Curcumin-loaded chitosan–cholesterol micelles: Evaluation in monolayers and 3D cancer spheroid model. Nanomedicine 2017, 12, 1435–1453.
- Muddineti, O.S.; Shah, A.; Rompicharla, S.V.K.; Ghosh, B.; Biswas, S. Cholesterol-grafted chitosan micelles as a nanocarrier system for drug-siRNA co-delivery to the lung cancer cells. Int. J. Biol. Macromol. 2018, 118, 857–863.
- Anirudhan, T.S.; Parvathy, J.; Nair, A.S. Evaluation of micellar architecture based on functionalized chitosan for the in vitro release of an antibiotic. Des. Monomers Polym. 2016, 19, 99–107.
- Xu, W.; Wang, H.; Dong, L.; Zhang, P.; Mu, Y.; Cui, X.; Zhou, J.; Huo, M.; Yin, T. Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int. J. Nanomed. 2019, 14, 4649–4666.
- Fu, D.-J.; Jin, Y.; Xie, M.-Q.; Ye, Y.-J.; Qin, D.-D.; Lou, K.-Y.; Chen, Y.-Z.; Gao, F. Preparation and characterization of mPEG grafted chitosan micelles as 5-fluorouracil carriers for effective anti-tumor activity. Chin. Chem. Lett. 2014, 25, 1435–1440.
- Zhao, X.; Yao, Y.; Tian, K.; Zhou, T.; Jia, X.; Li, J.; Liu, P. Leakage-free DOX/PEGylated chitosan micelles fabricated via facile one-step assembly for tumor intracellular pH-triggered release. Eur. J. Pharm. Biopharm. 2016, 108, 91–99.
- Chen, H.; Chen, Y.; Yang, H.; Xu, W.; Zhang, M.; Ma, Y.; Achilefu, S.; Gu, Y. A dual-targeting nanocarrier based on modified chitosan micelles for tumor imaging and therapy. Polym. Chem. 2014, 5, 4734–4746.
- Cheng, L.-C.; Jiang, Y.; Xie, Y.; Qiu, L.-L.; Yang, Q.; Lu, H.-Y. Novel amphiphilic folic acid-cholesterol-chitosan micelles for paclitaxel delivery. Oncotarget 2016, 8, 3315–3326.
- Liang, N.; Sun, S.; Gong, X.; Li, Q.; Yan, P.; Cui, F. Polymeric micelles based on modified glycol chitosan for paclitaxel delivery: Preparation, characterization and evaluation. Int. J. Mol. Sci. 2018, 19, 1550.
- Li, L.; Liangb, N.; Wang, D.; Yana, P.; Kawashimac, Y.; Cui, F.; Suna, S. Amphiphilic Polymeric Micelles Based on Deoxycholic Acid and Folic Acid Modified Chitosan for the Delivery of Paclitaxel. Int. J. Mol. Sci. 2018, 19, 3132.
- Qu, G.; Hou, S.; Qu, D.; Tian, C.; Zhu, J.; Xue, L.; Ju, C.; Zhang, C. Self-assembled micelles based on N-octyl-N’-phthalyl-O-phosphoryl chitosan derivative as an effective oral carrier of paclitaxel. Carbohydr. Polym. 2019, 207, 428–439.
- Yang, T.; Feng, J.; Zhang, Q.; Wu, W.; Mo, H.; Huang, L.; Zhang, W. l-Carnitine conjugated chitosan-stearic acid polymeric micelles for improving the oral bioavailability of paclitaxel. Drug Deliv. 2020, 27, 575–584.
- Han, Y.; Liangb, N.; Yana, P.; Kawashimac, Y.; Cui, F.; Suna, S. A Chitosan-Based Micellar System as Nanocarrier For the Delivery of Paclitaxel. Polymers 2020, 12, 380.
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570.
- Li, J.; Huo, M.; Wang, J.; Zhou, J.; Mohammad, J.M.; Zhang, Y.; Zhu, Q.; Waddad, A.Y.; Zhang, Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320.
- Šmejkalová, D.; Nešporová, K.; Hermannová, M.; Huerta-Angeles, G.; Čožíková, D.; Vištejnová, L.; Safrankova, B.; Novotny, J.; Kučerík, J.; Velebny, V. Paclitaxel isomerisation in polymeric micelles based on hydrophobized hyaluronic acid. Int. J. Pharm. 2014, 466, 147–155.
- Liu, J.; Liang, N.; Li, S.; Han, Y.; Yan, P.; Kawashima, Y.; Cui, F.; Suna, S. Tumor-targeting and redox-sensitive micelles based on hyaluronic acid conjugate for delivery of paclitaxel. J. Biomater. Appl. 2020, 34, 1458–1469.
- Deng, L.; Wang, G.; Ren, J.; Zhang, B.; Yan, J.; Li, W.; Khashab, N.M. Enzymatically triggered multifunctional delivery system based on hyaluronic acid micelles. RSC Adv. 2012, 2, 12909–12914.
- Saadat, E.; Amini, M.; Dinarvand, R.; Dorkoosh, F.A. Polymeric micelles based on hyaluronic acid and phospholipids: Design, characterization, and cytotoxicity. J. Appl. Polym. Sci. 2014, 131, 40944–40952.
- Qiu, L.; Zhu, M.; Huang, Y.; Gong, K.; Chen, J. Mechanisms of cellular uptake with hyaluronic acid—octadecylamine micelles as drug delivery nanocarriers. RSC Adv. 2016, 6, 39896–39902.
- Gao, Q.-Q.; Zhang, C.-M.; Zhang, E.-X.; Chen, H.-Y.; Zhen, Y.-H.; Zhang, S. Zwitterionic pH-responsive hyaluronic acid polymer micelles for delivery of doxorubicin. Colloids Surf. B Biointerfaces 2019, 178, 412–420.
- Wang, J.; Li, Y.; Wang, L.; Wang, X.; Tu, P. Comparison of hyaluronic acid-based micelles and polyethylene glycol-based micelles on reversal of multidrug resistance and enhanced anticancer efficacy in vitro and in vivo. Drug Deliv. 2018, 25, 330–340.
- Zheng, S.; Han, J.; Jin, Z.; Kim, C.-S.; Park, S.; Kim, K.-P.; Park, J.-O.; Choi, E. Dual tumor-targeted multifunctional magnetic hyaluronic acid micelles for enhanced MR imaging and combined photothermal-chemotherapy. Colloids Surf. B Biointerfaces 2018, 164, 424–435.
- Zhu, Y.; Zhang, J.; Meng, F.; Cheng, L.; Feijen, J.; Zhong, Z. Reduction-responsive core-crosslinked hyaluronic acid-b-poly(trimethylene carbonate-co-dithiolane trimethylene carbonate) micelles: Synthesis and CD44-mediated potent delivery of docetaxel to triple negative breast tumor in vivo. J. Mater. Chem. B 2018, 6, 3040–3047.
- Bongiovì, F.; Fiorica, C.; Palumbo, F.S.; Di Prima, G.; Giammona, G.; Pitarresi, G. Imatinib-Loaded Micelles of Hyaluronic Acid Derivatives for Potential Treatment of Neovascular Ocular Diseases. Mol. Pharm. 2018, 15, 5031–5045.
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881.
- Ahn, D.-G.; Lee, J.; Park, S.-Y.; Kwark, Y.-J.; Lee, K.Y. Doxorubicin-Loaded Alginate-g-Poly(N-isopropylacrylamide) Micelles for Cancer Imaging and Therapy. ACS Appl. Mater. Interfaces 2014, 6, 22069–22077.
- Yurong, G.; Li, G.; Gao, Y.; Jiang, H.; Tao, Q. Thermo-sensitive complex micelles from sodium alginate- graft -poly(N -isopropylacrylamide) for drug release. Int. J. Biol. Macromol. 2016, 86, 296–301.
- Wu, J.; Wu, Z.; Zhang, R.; Yuan, S.; Lu, Q.; Yu, Y. Synthesis and micelle properties of the hydrophobic modified alginate. Int. J. Polym. Mater. 2017, 66, 742–747.
- Tang, Y.; Chen, K.; Li, J.; Feng, Y.; Yu, G.; Wang, L.; Zhao, X.; Peng, Y.; Zhang, Q. Electrolyte and pH-sensitive amphiphilic alginate: Synthesis, self-assembly and controlled release of acetamiprid. RSC Adv. 2018, 8, 32193–32199.
- Song, Y.; Zhang, L.; Gan, W.; Zhou, J.; Zhang, L. Self-assembled micelles based on hydrophobically modified quaternized cellulose for drug delivery. Colloids Surf. B Biointerfaces 2011, 83, 313–320.
- Songsurang, K.; Siraleartmukul, K.; Muangsin, N. Mucoadhesive drug carrier based on functional-modified cellulose as poorly water-soluble drug delivery system. J. Microencapsul. 2015, 32, 450–459.
- Zhong, H.; Zhang, J.; Guo, Y.; Ge, W.; Sun, R.; Chen, M.-W.; Wang, X.; Wang, L. Multi-color light-emitting amphiphilic cellulose/conjugated polymers nanomicelles for tumor cell imaging. Cellulose 2016, 24, 889–902.
- Wang, H.; Li, Z.; Lu, S.; Li, C.; Zhao, W.; Zhao, Y.; Yu, S.; Wang, T.; Sun, T. Nano micelles of cellulose-graft-poly (l-lactic acid) anchored with epithelial cell adhesion antibody for enhanced drug loading and anti-tumor effect. Mater. Today Commun. 2020, 22, 100764.
- Sun, H.; Guo, B.; Li, X.; Cheng, R.; Meng, F.; Liu, H.; Zhong, Z. Shell-Sheddable Micelles Based on Dextran-SS-Poly(ε-caprolactone) Diblock Copolymer for Efficient Intracellular Release of Doxorubicin. Biomacromolecules 2010, 11, 848–854.
- Wu, X.; Chen, X.; Hu, P.; Hou, M.; Dong, Y.; Wei, Y. Antifouling zwitterionic dextran micelles for efficient loading DOX. Carbohydr. Polym. 2018, 191, 136–141.
- Du, Y.; Weng, Q.; Yuan, H.; Hu, F.-Q. Synthesis and Antitumor Activity of Stearate-g-dextran Micelles for Intracellular Doxorubicin Delivery. ACS Nano 2010, 4, 6894–6902.
- Varshosaz, J.; Hassanzadeh, F.; Sadeghi, H.M.M.; Firozian, F.; Mirian, M. Effect of Molecular Weight and Molar Ratio of Dextran on Self-Assembly of Dextran Stearate Polymeric Micelles as Nanocarriers for Etoposide. J. Nanomater. 2012, 2012, 265657.
- Lin, B.; Su, H.; Jin, R.; Li, D.; Wu, C.; Jiang, X.; Xia, C.; Gong, Q.; Song, B.; Ai, H. Multifunctional dextran micelles as drug delivery carriers and magnetic resonance imaging probes. Sci. Bull. 2015, 60, 1272–1280.
- Situ, J.-Q.; Wang, X.-J.; Zhu, X.-L.; Xu, X.-L.; Kang, X.-Q.; Hu, J.-B.; Lu, C.-Y.; Ying, X.-Y.; Yu, R.-S.; You, J.; et al. Multifunctional SPIO/DOX-loaded A54 Homing Peptide Functionalized Dextran-g-PLGA Micelles for Tumor Therapy and MR Imaging. Sci. Rep. 2016, 6, 35910.
- Verma, M.S.; Liu, S.; Chen, Y.Y.; Meerasa, A.; Gu, F.X. Size-tunable nanoparticles composed of dextran-b-poly(D,L-lactide) for drug delivery applications. Nano Res. 2011, 5, 49–61.
- Zhao, Z.; Zhang, Z.; Chen, L.; Cao, Y.; He, C.; Zhu, X. Biodegradable Stereocomplex Micelles Based on Dextran-block-polylactide as Efficient Drug Deliveries. Langmuir 2013, 29, 13072–13080.
- Jeong, Y.; Kim, D.H.; Chung, C.W.; Yoo, J.J.; Choi, K.H.; Kim, C.H.; Ha, S.H.; Kang, D.H. Doxorubicin-incorporated polymeric micelles composed of dextran-b-poly(DL-lactide-co-glycolide) copolymer. Int. J. Nanomed. 2011, 6, 1415–1427.
- Yu, J.; Zhou, Y.; Chen, W.; Ren, J.; Zhang, L.; Lu, L.; Luo, G.; Huang, H. Preparation, Characterization and Evaluation of α-Tocopherol Succinate-Modified Dextran Micelles as Potential Drug Carriers. Materials 2015, 8, 6685–6696.
- Wu, Y.; Wang, X. Binding, stability, and antioxidant activity of curcumin with self-assembled casein–dextran conjugate micelles. Int. J. Food Prop. 2017, 20, 3295–3307.
- Li, S.; Yi, J.; Li, W.; Wang, L.; Wang, Z. Synthesis and characterization of three novel amphiphilic dextran self-assembled micelles as potential drug delivery system. J. Mater. Sci. 2017, 52, 12593–12607.
- Blanco-Fernandez, B.; Concheiro, A.; Makwana, H.; Fernandez-Trillo, F.; Alexander, C.; Alvarez-Lorenzo, C. Dually sensitive dextran-based micelles for methotrexate delivery. RSC Adv. 2017, 7, 14448–14460.
- Das Karmakar, P.; Seesala, V.S.; Pal, A.; Dhara, S.; Chatterjee, S.; Pal, S. Synthesis of RAFT-Mediated Amphiphilic Graft Copolymeric Micelle Using Dextran and Poly (Oleic Acid) toward Oral Delivery of Nifedipine. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2354–2363.
- Fan, Y.; Picchioni, F. Modification of starch: A review on the application of “green” solvents and controlled functionalization. Carbohydr. Polym. 2020, 241, 116350.
- Zhang, A.; Zhang, Z.; Shi, F.; Ding, J.; Xiao, C.; Zhuang, X.; He, C.; Chen, L.; Zhu, X. Disulfide crosslinked PEGylated starch micelles as efficient intracellular drug delivery platforms. Soft Matter 2013, 9, 2224–2233.
- Wu, C.; Yang, J.; Xu, X.; Gao, C.; Lü, S.; Liu, M. Redox-responsive core-cross-linked mPEGylated starch micelles as nanocarriers for intracellular anticancer drug release. Eur. Polym. J. 2016, 83, 230–243.
- Wen, N.; Gao, C.; Lü, S.; Xu, X.; Bai, X.; Wu, C.; Ning, P.; Zhang, S.; Liu, M. Novel amphiphilic glucose-responsive modified starch micelles for insulin delivery. RSC Adv. 2017, 7, 45978–45986.
- Wen, N.; Lü, S.; Gao, C.; Xu, X.; Bai, X.; Wu, C.; Ning, P.; Liu, M. Glucose-responsive zwitterionic dialdehyde starch-based micelles with potential anti-phagocytic behavior for insulin delivery. Chem. Eng. J. 2018, 335, 52–62.
- Kou, Z.; Dou, D.; Zhu, J.; Mai, Y.; Yi, H.; Lan, L.; Lan, P. Release Mechanism and pH Responsiveness of Starch-Based Polymers. Nano 2019, 14, 1950145.
- Li, G.; Zhao, L. Sorafenib-loaded hydroxyethyl starch-TG100-115 micelles for the treatment of liver cancer based on synergistic treatment. Drug Deliv. 2019, 26, 756–764.
- Lin, Q.; Liang, R.; Zhong, F.; Ye, A.M.; Hemar, Y.; Yang, Z.; Singh, H. Self-Assembled Micelles Based on OSA-Modified Starches for Enhancing Solubility of β-Carotene: Effect of Starch Macromolecular Architecture. J. Agric. Food Chem. 2019, 67, 6614–6624.
- Maiti, S.; Mukherjee, S. Controlled drug delivery attributes of co-polymer micelles and xanthan-O-carboxymethyl hydrogel particles. Int. J. Biol. Macromol. 2014, 70, 37–43.
- Garhwal, R.; Shady, S.F.; Ellis, E.J.; Ellis, J.Y.; Leahy, C.D.; McCarthy, S.P.; Crawford, K.S.; Gaines, P. Sustained Ocular Delivery of Ciprofloxacin Using Nanospheres and Conventional Contact Lens Materials. Investig. Opthalmol. Vis. Sci. 2012, 53, 1341–1352.
- Wang, J.; Cui, S.; Bao, Y.; Xing, J.; Hao, W. Tocopheryl pullulan-based self assembling nanomicelles for anti-cancer drug delivery. Mater. Sci. Eng. C 2014, 43, 614–621.
- Chen, L.; Wang, X.; Ji, F.; Bao, Y.; Wang, J.; Wang, X.; Guo, L.; Li, Y. New bifunctional-pullulan-based micelles with good biocompatibility for efficient co-delivery of cancer-suppressing p53 gene and doxorubicin to cancer cells. RSC Adv. 2015, 5, 94719–94731.
- Hassanzadeh, F.; Varshosaz, J. Biotin-encoded Pullulan-Retinoic Acid Engineered Nanomicelles: Preparation, Optimization and In Vitro Cytotoxicity Assessment in MCF-7 Cells. Indian J. Pharm. Sci. 2016, 78, 557–565.
- Chen, L.; Ji, F.; Bao, Y.; Xia, J.; Guo, L.; Wang, J.; Li, Y. Biocompatible cationic pullulan-g-desoxycholic acid-g-PEI micelles used to co-deliver drug and gene for cancer therapy. Mater. Sci. Eng. C 2017, 70, 418–429.
- Hassanzadeh, F.; Mahmoudi, E.; Varshosaz, J.; Khodarahmi, G.A.; Rostami, M.; Ghanadian, M.; Dana, N. Novel NGR anchored pullulan micelles for controlled and targeted delivery of doxorubicin to HeLa cancerous cells. Iran. Polym. J. 2018, 27, 263–274.
- Constantin, M.; Bucatariu, S.; Ursu, L.; Butnaru, M.; Daraba, O.M.; Burlui, A.M.; Fundueanu, G. Novel Cationic and Hydrophobic Pullulan Derivatives as DNA Nanoparticulate Carriers. Cellul. Chem. Technol. 2019, 53, 695–707.
- Yuan, H.; Zhong, W.; Wang, R.; Zhou, P.; Nie, Y.; Hu, W.; Tao, X.; Yang, P. Preparation of Cholesteryl-Modified Aminated Pullulan Nanoparticles to Evaluate Nanoparticle of Hydrophobic Degree on Drug Release and Cytotoxicity. J. Nanomater. 2020, 2020, 1–10.
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Synthesis and characterization of redox-sensitive heparin-β-sitosterol micelles: Their application as carriers for the pharmaceutical agent, doxorubicin, and investigation of their antimetastatic activities in vitro. Mater. Sci. Eng. C 2017, 75, 1326–1338.
- Emami, J.; Kazemi, M.; Hasanzadeh, F.; Minaiyan, M.; Mirian, M.; Lavasanifar, A. Novel pH-triggered biocompatible polymeric micelles based on heparin–α-tocopherol conjugate for intracellular delivery of docetaxel in breast cancer. Pharm. Dev. Technol. 2020, 25, 492–509.
- Peng, N.; Yang, M.; Tang, Y.; Zou, T.; Guo, F.; Wu, K.; Wang, X.; Li, X.; Li, X. Amphiphilic hexadecyl-quaternized chitin micelles for doxorubicin delivery. Int. J. Biol. Macromol. 2019, 130, 615–621.
- Cerqueira, M.A.; Pinheiro, A.C.; Pastrana, L.M.; Vicente, A.A. Amphiphilic Modified Galactomannan as a Novel Potential Carrier for Hydrophobic Compounds. Front. Sustain. Food Syst. 2019, 3, 17.
- Zhang, G.; Huang, L.; Wu, J.; Liu, Y.; Zhang, Z.; Guan, Q. Doxorubicin-loaded folate-mediated pH-responsive micelle based on Bletilla striata polysaccharide: Release mechanism, cellular uptake mechanism, distribution, pharmacokinetics, and antitumor effects. Int. J. Biol. Macromol. 2020, 164, 566–577.
- Oliveira, A.C.D.J.; Chaves, L.L.; Ribeiro, F.D.O.S.; De Lima, L.R.M.; Oliveira, T.C.; García-Villén, F.; Viseras, C.; De Paula, R.C.; Rolim-Neto, P.J.; Hallwass, F.; et al. Microwave-initiated rapid synthesis of phthalated cashew gum for drug delivery systems. Carbohydr. Polym. 2021, 254, 117226.
- Negahban, Z.; Shojaosadati, S.A.; Hamedi, S. A novel self-assembled micelles based on stearic acid modified schizophyllan for efficient delivery of paclitaxel. Colloids Surf. B Biointerfaces 2021, 199, 111524.
- Shu, G.; Lu, C.; Wang, Z.; Du, Y.; Xu, X.; Xu, M.; Zhao, Z.; Chen, M.; Dai, Y.; Weng, Q.; et al. Fucoidan-based micelles as P-selectin targeted carriers for synergistic treatment of acute kidney injury. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102342.
- Cao, C.; Zhao, J.; Lu, M.; Garvey, C.J.; Stenzel, M.H. Correlation between Drug Loading Content and Biological Activity: The Complexity Demonstrated in Paclitaxel-Loaded Glycopolymer Micelle System. Biomacromolecules 2019, 20, 1545–1554.
