1. Introduction
Biosurfactants (BSs) are a structurally heterogeneous group of biomolecules that share pronounced surface and emulsifying activities. They can be either located on microbial cell surfaces or released in the extracellular space by different bacteria (
Bacillus,
Lactobacillus,
Pseudomonas, Burkholderia, Mycobacterium, Rhodococcus, Arthrobacter, Nocardia, Gordonia and
Acinetobacter), yeast and filamentous fungi (
Candida, Saccharomyces,
Starmerella, Trichosporon, Pseudozyma and
Ustilago) [
1,
2]. They are, therefore, mostly classified by their structural features, the producing microorganisms and their molecular mass. BSs have a hydrophilic region (carbohydrate, amino acid, cyclic peptide, phosphate, carboxylic acid or alcohol) and a hydrophobic region (saturated, unsaturated, linear, or branched long-chain fatty acids or hydrocarbon acids). This amphipathic structure allows a reduction in surface tension at the interfaces of phases with dissimilar polarities (liquid–air, liquid–liquid or liquid–solid) [
3,
4]. They have the ability to form molecular aggregates, including micelles. The micellar aggregation of BSs is originated at the critical micelle concentration (CMC) typically from 1 to 200 mg/L and, interestingly, about 10- to 40-fold lower than that of chemical surfactants [
5].
Based on their molecular weight, BSs are commonly divided into two main classes: the low molecular weight compounds efficiently lower surface tension and interfacial tension and are appropriately called “biosurfactants”; conversely, the high molecular weight polymers are more effective as emulsion-stabilizing agents and are usually called “bioemulsifiers”. According to the chemical composition, BSs can be classified into five major groups: glycolipids, lipopeptides, phospholipids, polymeric compounds and neutral lipids [
6].
The most widely studied groups of BSs are lipopeptides, such as surfactin, fengycin and iturin, and glycolipids, such as rhamnolipids, sophorolipids, mannosylerythritol lipids and trehalose lipids [
7]. Since the 1980s, these amphipathic molecules have been extensively applied in the biodegradation and detoxification of industrial effluents, bioremediation, industrial emulsions and enhanced oil recovery due to their emulsification, wetting, foaming, cleansing, phase separation, surface activity and reduction in heavy liquid viscosity [
8,
9,
10].
BSs might present valuable alternatives to petroleum-based surfactants. Additional advantageous properties, emphasizing the uniqueness of these natural molecules, include the possibility to modify their chemical composition through genetic engineering or the use of biological and biochemical techniques to alter the metabolic end products, thus tailoring them to meet specific functional requirements [
11,
12]. In addition, they are claimed to be more biodegradable and eco-friendly than synthetic surfactants [
13,
14,
15,
16], less toxic and effective even at extremes temperatures, pH conditions, and salinity [
6,
13,
17,
18,
19].
Despite having a large number of advantages, some disadvantages are also linked to biosurfactants, such as high production cost and the need for purification for some specific applications (e.g., pharmaceutical). Biotechnological processes involved in the synthesis of biosurfactants are rather expensive, and the purification of surfactants is problematic. Several research groups are engaged in finding a solution for cost reductions in biosurfactant production by using easily available and renewable bioresources as cheap raw materials, industrial wastes or by-products [
15].
In terms of biodegradability, as water-soluble molecules, BSs may be susceptible to fast biodegradation by other microorganisms, thus limiting hydrocarbon degradation during bioremediation [
20]. Additionally, it is also important to remark that for many applications, especially in biomedical and pharmaceutical processes, it would be interesting if biosurfactants were not biodegraded immediately to develop their function in the formulations where they have been included. However, from an environmental point of view, it could represent a problem, not only because of the changes in microbiota caused by the antimicrobial effect of biosurfactants but also due to the costs that could imply their exclusion [
21]. Consequently, it is necessary to study the biodegradation process of biosurfactants to establish not only their environmental impact but also to determine their optimal formulation conditions and stability when applied in different industrial sectors [
22].
In addition, critical “Life Cycle Assessment”, which typically considers industrial processes from the basic acquisition of raw materials, to the manufacturing of products, consumer use and, finally, the disposal of waste materials. Such approach does not fundamentally show that a biosurfactant has a much lower environmental impact, in terms of greenhouse gas emissions, than petrochemically derived surfactant processes [
11].
Studies for potential applications of biosurfactants in the medical field have increased during the past decade; the pertinence in these fields is mostly related to their biological properties, such as their ability to affect cell membrane permeability, emulsification and adhesion to biotic and abiotic surfaces.
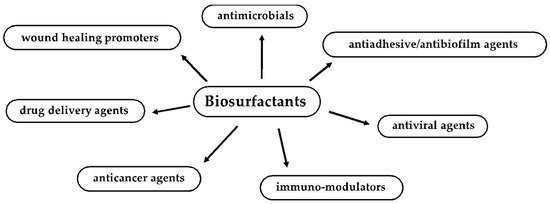
This review focuses on recent advances in the understanding of BSs’ antimicrobial, antiviral, antiadhesive, antibiofilm, wound healing, anticancer and immune-modulatory activities and their promising application in the field of human health [
18,
23,
24,
25] (). Some critical issues related to the production and application of these molecules will also be presented.
Figure 1. Biomedical, therapeutic and pharmaceutical applications of biosurfactants.
2. Biosurfactant Properties and Biological Activities Useful for Biomedical and Pharmaceutical Applications
In nature, BSs modulate various biological activities, including microbial metabolism, motility and survival. These molecules increase the surface areas and bioavailability of hydrophobic water-insoluble substrates and are responsible for the removal of heavy metals from the surrounding environment. They also regulate the attachment/detachment of microorganisms to and from surfaces, mobilization, cell surface conditioning, aggregation at interfaces and surfaces on which the interaction takes place. In addition, cellular differentiation, substrate accession and resistance to toxic compounds are all roles attributed to microbial surface-active compounds [
26]. Rhamnolipids, for example, play multiple roles in the survival of microorganisms. They are crucial for the preservation of biofilm architecture and are considered as one of the virulence factors in
Pseudomonas sp. [
27,
28] and as part of a natural mechanism evolved to improve the uptake of hydrophobic substrates by bacterial cells. However, current evidence confirms that rhamnolipids are part of a mechanism which controls the fundamental elements of microbial existence, such as the stimulation of bacterial motility, formation and disruption of biofilms, virulence and antimicrobial activity [
29].
Overall, BSs confer a selective advantage to the producer microorganism; consequently, they exert antimicrobial activity against other microorganisms that do not produce BSs. BSs can act as virulence factors and as quorum-sensing molecules, regulating the expression of other virulence factors, such as those promoting biofilm formation, maintenance and, ultimately, biofilm dispersal. In addition, they are crucial in maintaining channels for gas and nutrient exchange across, and diffusion into, the biofilm surface and structure [
26,
27,
30,
31,
32].
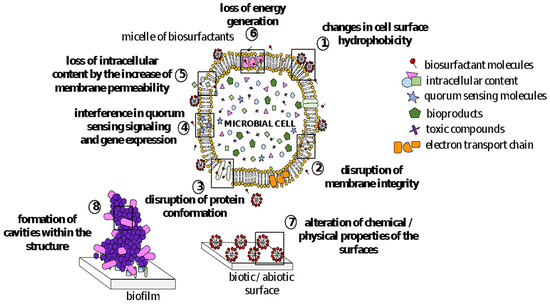
In recent years, a growing number of studies have pointed out that BSs harbor many biological properties exploitable by biomedical and pharmaceutical fields. BSs mechanism of action on microbial cell surfaces involves binding/attachments to membranes, causing changes in wettability and surface energy, leading to a reduction in hydrophobicity and an increase in permeability through the release of LPS and the formation of transmembrane pores. They, therefore, disrupt membrane integrity, leading to cell lysis and metabolite leakage; loss of membrane functions, such as transport and energy generation processes; and disruption of protein structures () [
7,
33,
34]. Several reports have suggested that, in addition to their direct action against pathogens, biosurfactants are able to interfere with biofilm formation, modulating microbial interaction with interfaces [
26] due to changes in surface tension and bacterial cell-wall charge [
35].
Figure 2. Mechanisms of action of biosurfactants against microbial cell membranes and biofilms.
It is envisaged that more in-depth studies of the natural role of BSs in microbial competitive interactions, cell-to-cell communication, pathogenesis, motility and biofilm formation and maintenance will improve and suggest many other interesting potential applications [
5].