Recently, the intranasal route has emerged as a promising administration site for central nervous system therapeutics since it provides a direct connection to the central nervous system, avoiding the passage through the blood–brain barrier, consequently increasing drug cerebral bioavailability.
- stimuli-responsive hydrogel
- intranasal administration
- nose to brain
- neurodegenerative diseases
- Alzheimer’s disease
- Parkinson’s disease
- drug delivery
1. Introduction
Neurological disorders represent the first cause of disability and the second cause of death, around 17%, after cardiovascular diseases. Unfortunately, in the following years, the number of patients suffering from neurological disease is expected to rise dramatically due to the increase in average life expectancy, thus causing a heavy global burden [1,2,3].
The most frequent neurologic diseases are Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, dementia, epilepsy, schizophrenia, stroke, and brain cancer. Although neurological disorders present different clinical manifestations and pathogenesis, they all mainly determine a progressive neuronal degeneration. Their etiology is complex and not completely known; however, genetic and environmental factors and aging are considered to have a key role [4,5,6,7].
Current therapeutic approaches against neurological disorders include oral, topical, or intravenous administration of drugs and more invasive techniques such as surgery, brain implants (i.e., deep brain stimulations), and so on. However, unfortunately, the currently available treatments are not associated with a repair and/or regeneration of the neural tissue and, consequently, resolution of the clinical situation but primarily act on alleviating symptoms and slowing the neurodegenerative process [8,9].
The main limitation of central nervous system therapeutics is related to their delivery to the nervous system in therapeutic quantities. In this regard, the blood–brain barrier (BBB) plays a key role in preventing the passage of many substances, including drugs, to the brain [10,11].
The BBB is a complex vascular system consisting of endothelial cells interconnected by extended tight junctions without fenestrations, whose main function is to maintain the nervous system homeostasis. The endothelial cells are surrounded by pericytes and astrocytes that contribute to the structural and functional preservation of the BBB.
The BBB is the main structure responsible for the protection of the central nervous system from circulating xenobiotics, which could have harmful effects on the neural function, and of the supplying of required nutrients to the neural tissue [12,13].
The main pathways for crossing the BBB are passive diffusion for small lipophilic compounds, active or passive transport for specific hydrophilic and/or ionized molecules (i.e., glucose, amino acids), and, lastly, for high-molecular-weight biological molecules, such as proteins and peptides, endocytosis [14,15]. The high selectivity of these crossing pathways massively prevents the entrance of circulating xenobiotics into the central nervous system, and for this reason, it has been estimated that around 98% of drugs is not able to cross the BBB [16].
In order to overcome these limitations, the intranasal route has recently emerged as a promising administration site for central nervous system therapeutics [17,18]. In fact, it is a noninvasive route that provides a direct connection to the central nervous system, nose-to-brain route, via neural pathways, such as the olfactory and trigeminal ones, avoiding the passage through the BBB and, consequently, increasing drug concentration at the brain level [19,20] (Figure 1).
Figure 1. Schematic representation of intranasal route for drug delivery to the brain.
Moreover, the intranasal route shows also the advantage, compared to the oral route, to prevent the gastrointestinal and first pass degradation, thus improving drug bioavailability [21].
2. Nasal Cavity: Anatomy and Physiology
The nose is the organ responsible for respiration and olfaction. Structurally, it is divided into the external nose and internal nose (nasal cavities).
The external nose is formed by bones and cartilages; it is placed in the center of the face and has the shape of a triangular pyramid. Small muscle groups controlled by the facial nerve that contribute to facial expression are connected to the external nose [22].
The nasal cavity extends around 12 cm in length from the external nose to the nasopharynx, and it is separated in two sections (left and right) by the nasal septum.
Structurally and functionally the nasal cavity can be divided into three regions: vestibular, olfactory, and respiratory [23].
The vestibular region is the smallest and outermost part of the nasal cavity lined firstly by stratified squamous epithelium followed by respiratory epithelium (pseudotratified columnar epithelium). It contains nasal hairs, called vibrissae, whose main function is to filter inhaled airborne particles, and sweat and sebaceous glands. This region can be considered irrelevant for drug absorption due to its structural features [7,24].
The respiratory region has the largest surface area and represents around 80% of the nasal cavity; in fact, it covers three turbinates, bones that extend laterally from each nasal cavity [25].
It is lined by pseudostratified columnar epithelium and contains four cell types: ciliated, nonciliated, basal, and goblet cells.
The respiratory region is covered by a thick layer of mucus produced by nasal glands and basal cells that plays a key role in trapping inhaled particles, preventing their entrance into the respiratory system. Ciliated cells contribute to this defense mechanism, called mucociliary clearance, transporting mucus to the nasopharynx where it is expectorated or ingested [26].
This region is highly vascularized, since it is supplied by a branch of the maxillary artery and it is mainly innervated by the trigeminal nerve [27].
The nasal region can be considered the main site for systemic drug absorption due to its large surface area, high vascularization, and viscosity and can contribute to the nose-to-brain delivery of drugs via the trigeminal pathway [28].
The olfactory region is located in the superior part of the nasal cavity (which represents about 10% of the nasal cavity area), and it is responsible for the olfaction. It is lined by pseudostratified columnar epithelium and contains four different cell types: basal, sustentacular, trigeminal, and olfactory neural cells [29].
Sustentacular cells are prevalent in the olfactory region and surround the olfactory neural cells, providing structural and metabolic support [27]. Olfactory neural cells are bipolar neurons that extend their dendritic processes into the mucus layer, terminating as olfactory receptors, and project into the olfactory bulb, providing a direct portal between the nose and the central nervous system. In particular, their unmyelinated axons are covered by olfactory ensheathing cells and olfactory nerve fibroblasts that are in continuity with meninges and, consequently, with the subarachnoid space [30].
The basal lamina is located below the epithelium and contains blood and lymphatic vessels, nerve fibers, and Bowman’s glands responsible for the secretion of mucus that, in turn, solubilizes odor substances, cleans sensory receptors, and traps xenobiotics [8]. The olfactory region is mainly supplied by a branch of the olfactory artery.
2.1. Nose-To-Brain Delivery Pathways
2.1.1. The Olfactory Pathway
2.1.2. The Trigeminal Pathway
3. Stimuli-Responsive Hydrogels
Hydrogels are three-dimensional networks formed by crosslinked hydrophilic polymers capable of absorbing large amounts of water. Recently, they have been studied as potential delivery platforms for biomedical applications due to their biocompatibility, biodegradability, nonimmunogenicity, and tunable properties [63].
Hydrogels can be divided into physical or chemical gels depending on the type of crosslinking that can be based on covalent bonds or weak physical interactions. In particular, physical crosslinking shows the advantage of obtaining hydrogels with tunable properties and responsivity to different external cues such as pH, temperature, and ionic modulation [64]. In this regard, in order to overcome the limitations of the intranasal route, stimuli-responsive hydrogels have emerged as an interesting strategy for the intranasal administration of drugs thanks to their ability to increase drug retention time and bioavailability and decrease mucociliary clearance [65]; in fact, they can be administered as liquid formulation, guaranteeing an accurate administration, and then, after instillation, they undergo sol–gel transition triggered by external physiological factors such as pH, temperature, ionic modulation, etc., leading to an increase of drug retention time and protection from enzymatic degradation [7].
Several mucoadhesive polymers such as chitosan, pluronic, carbopol, and cellulose derivatives exhibit sol–gel behavior in response to several cues and, consequently, can be used for stimuli-responsive hydrogels preparation in order to further improve retention time and drug absorption [66].
Moreover, the use of hydrogels as drug delivery systems shows the advantage to obtain a high drug-loading efficiency and sustained release, thus reducing the dose frequency and improving patient compliance and, thanks to their viscosity, a prolonged retention time [67].
Stimuli-responsive hydrogels can be divided in several classes depending on the external cues, and among them, thermos-, pH-, and ion-responsive gels are the more efficient delivery platforms for intranasal administration [68].
Thermoresponsive gels show sol–gel transition in response to a specific temperature change. Ideally, the temperature range should be at physiological values around 25–37 °C in order to guarantee an easy and accurate administration and avoid early drug loss [7].
Among thermoresponsive polymers, poloxamer, in particular poloxamer 407 and 188, is the most frequently used gelling agent for the preparation of in situ gels thanks to its mucoadhesiveness and sol–gel transition at physiological temperature values. It is a triblock polymer composed of one unit of polyoxypropylene and two units of polyoxyethylene that undergo sol–gel transition driven by supramolecular entanglements of micelle consequent to the dehydration of the polyoxypropylene units [69]. However, poloxomer-based hydrogels show low mechanical strength and viscosity in physiological conditions that limit their use, and, consequently, in order to overcome this problem, usually poloxamer is mixed with other polymers such as carbopol, chitosan, cellulose derivatives, etc. [70,71,72].
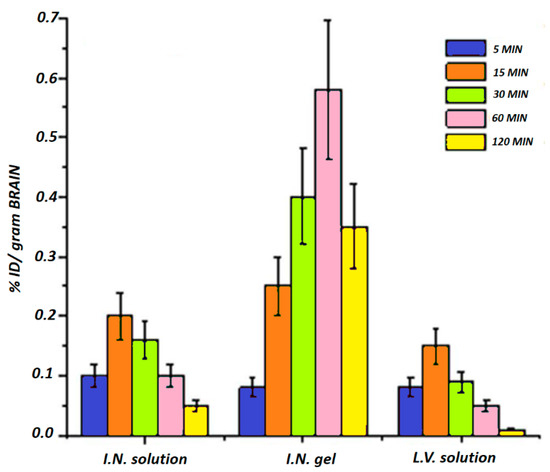
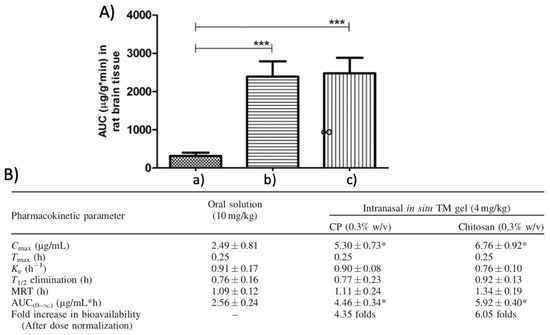
In this context, Ahmed et al. designed a poloxamer-/chitosan-based thermoresponsive gel loaded with agomelantine and evaluated its suitability and efficacy for the delivery of antidepressant drugs via the nose-to-brain route [73]. Firstly, the hydrogel was prepared by physically mixing chitosan (0.5% w/v), an agomelantine-based nanoemulsion and poloxamer 407 (20% w/v) (Figure 3). Chitosan was added in order to increase the mucoadhesiveness of the formulation; in fact, the calculated mucoadhesive strengths were 3747.75 dynes/cm2 and 6246.27 dynes/cm2 for the formulation without and with chitosan, respectively, and, moreover, the obtained hydrogel showed high strength and viscosity, advantageous features that further enhance drug retention time and adsorption. In fact, the pharmacokinetics studies highlighted a ~three-fold higher drug bioavailability in the brain, when administered intranasally compared to the intravenous administration of the drug solution.
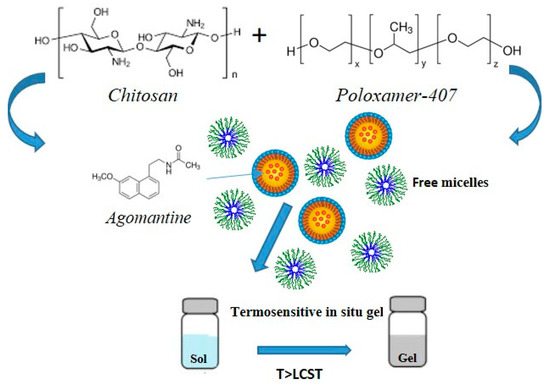
In addition to poloxamer, other polymers such as chitosan usually mixed with polyols, cellulose derivatives like ethyl hydroxy-ethyl cellulose, and xyloglucan are used as gelling agents for the preparation of thermoresponsive hydrogels [74].
3.1. Stimuli-Responsive Hydrogel for the Treatment of Alzheimer’s Disease
3.2. Stimuli-Responsive Hydrogels for the Treatment of Parkinson’s Disease
3.3. Stimuli-Responsive Hydrogels for the Treatment of Depression
3.4. Stimuli-Responsive Hydrogels for the Treatment of Schizophrenia
3.5. Stimuli-Responsive Hydrogels for the Treatment of Brain Injury
Stroke is the second cause of death worldwide and one of the major causes of disability. It is caused by impaired perfusion and blockage of cerebral blood vessels that lead to hypoxia and, consequently, cell death and cerebral damage. Stroke can be ischemic or hemorrhagic: ischemic stroke is caused by blood vessels occlusion and usually results in thrombotic and embolic conditions, while hemorrhagic stroke is caused by blood vessels rupture, and it is associated with higher mortality.
At present, therapeutic approaches are limited and involve mainly reperfusion treatments such as intravenous thrombolysis [107]. However, there are many natural and synthetic agents that have gained attention due to their neuroprotective effect; in this regard, Xie et al. developed an intranasal formulation combining polyamidoamine dendrimers with an in situ gellan-bum-based gel for the nose-to-brain delivery of paenol, a neuroprotective agent that was found to reduce cerebral stroke in murine models. Firstly, polyamidoamine dendrimers were prepared and loaded with paenol obtaining an encapsulation efficiency of ~54%, and, subsequently, incorporated in an ion-sensitive gel obtained by using gellan gum 0.45% w/v and hydroxypropyl cellulose 0.3% w/v via cold method. The developed formulation showed advantageous features such as low critical ion concentration, facilitating the sol–gel transition under physiological condition, adequate viscosity and strength guaranteeing easy of spraying, and sustained release profile with ~80% of the drug released within 12 h. Moreover, fluorescence studies investigating the nose-to-brain delivery mechanism were performed and highlighted a greater cerebral bioavailability of paenol after the intranasal administration of the gel compared to the drug solution [108].
This entry is adapted from the peer-reviewed paper 10.3390/ma14071802
