The coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was proclaimed a global pandemic in March 2020. Reducing the dissemination rate, in particular by tracking the infected people and their contacts, is the main instrument against infection spreading. Therefore, the creation and implementation of fast, reliable and responsive methods suitable for the diagnosis of COVID-19 are required. These needs can be fulfilled using affinity sensors, which differ in applied detection methods and markers that are generating analytical signals. Recently, nucleic acid hybridization, antigen-antibody interaction, and change of reactive oxygen species (ROS) level are mostly used for the generation of analytical signals, which can be accurately measured by electrochemical, optical, surface plasmon resonance, field-effect transistors, and some other methods and transducers.
- COVID-19
- SARS-CoV-2 virus
- RNA analysis
- bioelectrochemistry
- biosensors
- electrochemical immunosensors
- antigen-antibody interaction
- immune complex
- molecularly imprinted polymers (MIPs)
- surface modification by immobilization of biomolecules
1. Introduction
The spreading of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is causing coronavirus disease 2019 (COVID-19) was declared as a global pandemic in March 2020. The main threat of the pandemic is the overloading of the health systems. The key tool against infection spreading is decreasing its distribution rate, in particular by monitoring the infected people and their contacts. For the successful control, the primary step is the detection of SARS-CoV-2 in an organism. Hence, the development and introduction of rapid, precise, and sensitive detection methods are required. For a better understanding of the existing detection method principles, it is worth dwelling in more detail on the structure of SARS-CoV-2, its life cycle and the induced host response.
2. Affinity Biosensors for COVID-19 Diagnosis
SARS-CoV-2 infection can be identified using affinity biosensors, some of which were reviewed recently [1]. Several different types of signal transduction systems can be applied, which include electrochemical, optical, piezoelectric and some others. Electrochemical affinity biosensors are the most prevalent in biomedical applications due to their cheapness, ease and facility of mass manufacture [2].
2.1. Affinity Biosensors for the Determination of SARS-CoV-2 RNA
An electrochemical DNA/RNA biosensor employs the hybridization of single-stranded nucleic acid (NA) with the complementary strand as a source of the electrochemical signal [3]. The affinity biosensor includes a biorecognition element consisting of the capture NA specifically interacting with the target NA, and the signal transducer where the identification event is transformed into an electrical signal [4] (Figure 1). Sometimes additional reporter probes, which are marked with signaling compounds, are used.
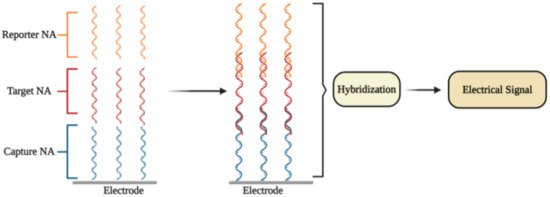
Figure 1. The general principle of electrochemical affinity biosensors for the detection of specific nucleic acid sequences [1].
In some electrochemical sensors, NA hybridization [3] includes an electrochemical reaction, which is further used for the quantitation of the detected NA fragment concentration and thus to the concentration of SARS-CoV-2 virus.
The selective identification of a low amount of DNA and/or RNA copies in specimens is the most important task for electrochemical NA biosensors. The choice of the most efficient signal amplification method is the key aspect that is used to resolve this task. The molecular approaches are classified into (1) NA-based amplification methods (enzyme-mediated isothermal amplification of NA), (2) nanomaterials-based methods (large surface area for the loading of capture NA; nanomaterials as reporter probes), and (3) enzyme-mediated signal amplification (enzymes are connected with NA hybridization system) [5]. Different electrochemical methods are employed for the quantitation of amplified signals, namely, electrochemical impedance spectroscopy (EIS) [6,7], chronoamperometry [8], pulsed amperometric detection [3,9], square wave voltammetry [10], differential pulse voltammetry (DPV) [11], and cyclic voltammetry (CV) [12,13].
2.1.1. DPV-Based Affinity Biosensors
Some researchers presented ultrasensitive DPV-based detection technology using calixarene functionalized graphene oxide for targeting RNA of SARS-CoV-2 [14]. It was affirmed that the technology identifies RNA of SARS-CoV-2 avoiding amplification and reverse transcription stages by employing a portable electrochemical smartphone. The biosensor consists of a capture probe, target sequence, label probe, and an auxiliary probe [15]. The capture probe is complementary to the 5′-terminal of the target sequence, while the label probe is complementary to the 3′-terminal; two different label probe areas have complementary sequences to 5′- and 3′-regions of the auxiliary probe [15,16]. The LOD in the clinical sample is 200 copies/mL, from which it follows that only two copies (10 μL) of all viral RNA copies are needed per analysis. The sensitivity for samples from confirmed COVID-19 patients was 85.5% [14].
It is worth noting that there are some investigations concerning the potential use of G-quadruplex-based biosensors in COVID-19 diagnosis [17]. G-quadruplex (GQ) is a guanine-rich DNA/RNA sequence, which is folded into four-stranded secondary structures. Recently, 25 putative G-quadruplex-forming sequences (PQSs) in the genome of SARS-CoV-2 virus were recognized [18]. The PQSs are situated in the ORF1ab, ORF3a, S-, M-, and N-genes of SARS-CoV-2 [17]. Some of the found PQSs are observed in a wide range of coronaviruses, while the main two PQSs, which generate RNA G-quadruplex structures, are strictly observed only in a limited range of viruses. Moreover, a straight interaction between G-quadruplex of coronavirus and viral helicase (nsp13) was obtained by microscale thermophoresis. The results of molecular docking-based modeling suggest that nsp13 alters the G-quadruplex structure. The helicase allows the guanine bases to go out of the guanine quartet planes, therefore, simplifying their unfolding [18]. Thus, RNA G-quadruplex sequences of SARS-CoV-2 could be used for the design of affinity-sensors, which are based on the identification of the viral helicase protein, nsp13 [18].
Fluorescence quenching is a powerful technique for the design of affinity biosensors [19]. One type of biosensor for the determination of enzymes based on fluorescence quenching by G-quadruplex has been reported recently [20].
2.1.2. Plasmonics-Based Affinity Biosensors
The primary concept of plasmonic biosensors is based on the distribution of surface plasmons lengthwise the interface of the thin metallic layer (usually noble metals), and dielectric [21]. Most plasmonic biosensors are built on the basics of surface plasmon resonance (SPR) [21,22]. Interactions occur on the surface that is suitable for observation SPR-based signals in two different modes: (1) bulk SPR signal and (2) localized SPR (LSPR) signal. Both effects rely on the refractive index of the ambient media to evoke spectral shifts.
It was reported that a dual-functional plasmonic biosensor incorporating the plasmonic photothermal (PPT) effect and LSPR sensing transmission enables the development of an alternative approach for SARS-CoV-2 virus detection, where the detection is provided through the hybridization of complementary NA with one NA immobilized on the surface of the gold nanoislands (AuNIs). The LSPR and PPT effects were utilized mutually to increase the signal. The LOD of this assay for the RdRp gene was 0.22 pM. The specificity, the discrimination between the RdRp gene of SARS-CoV and SARS-CoV-2, can be precisely established by onsite PPT improvement on gold AuNIs-based chips [23]. Plasmonic biosensing has technological benefits including the possibility of a combination of SPR with electrochemical, and electroassisted chemiluminescence methods [24].
2.2. Immunosensors for Determination of SARS-CoV-2 Proteins
2.2.1. Field-Effect Transistor Based Immunosensors
It was reported that a Field-effect transistor (FET)-based biosensor enables the real-time detection of SARS-CoV-2 in clinical specimens. The device was manufactured by covering the graphene plates of the FET with an antibody produced as a response to the SARS-CoV-2 S-protein. The antibody was fixed on the surface of the biosensor by 1-pyrenebutyric acid N-hydroxysuccinimide ester (PBASE) (Figure 2). The cultured virus, antigen protein, and nasopharyngeal swab samples from an infected person have been utilized for the assessment of the efficiency of the immunosensor. It was determined that the FET immunosensor enables the detection of the S-protein at a concentration of 1 fg/mL in PBS and 100 fg/mL in the transport medium, whereas LOD for SARS-CoV-2 was 1.6 × 101 pfu/mL in culture medium and 2.42 × 102 copies/mL in clinical specimens [25].
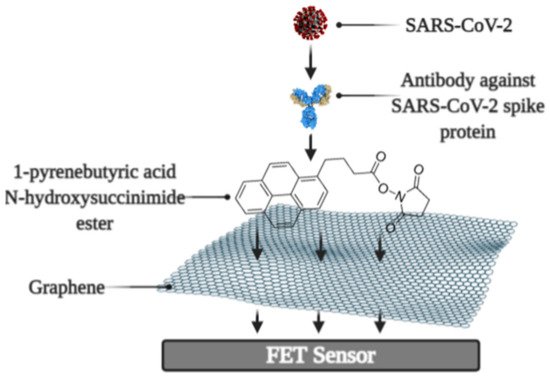
Figure 2. Schematic representation of field-effect transistor-based immunosensor for SARS-CoV-2 detection [1].
2.2.2. Quartz Crystal Microbalance Based Biosensors
The quartz crystal microbalance (QCM) can be successfully applied for the development of affinity biosensors [26]. In QCM-based approach, the binding with the viral S-protein occurs on the engineered quartz crystal surface covered by self-assembled monolayer (SAM), and the detection is carried out by QCM. The main working principle of the QCM is altering (decreasing) the frequency of the vibrating quartz crystal with the increasing the adsorbed mass [27].
One more type of affinity biosensor, which is very promising for the determination of virus-induced diseases, is the ultrasound transducer-based immunosensors, e.g.: capacitive micromachined ultrasound transducer (cMUT) was applied in immunosensors for the detection of specific antibodies against some virus proteins [28]. Moreover, the ultrasound-based test allows performing the SARS-CoV-2 virus detection in the gas phase (ultrasonator-produced viral aerosol) [29], while the vast majority of the assays are designed for the solution.
2.2.3. Molecularly Imprinted Polymer Based Electrochemical Affinity Sensors
In affinity sensors, the target protein is detected on the surface of the device, thus the design of the surface with appropriate protein recognition properties is required for the development of such sensors. For this purpose, molecularly imprinted polymers (MIPs) can be very efficiently applied [7,30-33]. The advantage of molecularly imprinted sensors is that they are cheaper and more stable, and can be based on protein-imprinted polymers such as polypyrrole [34] and some other electrochemically deposited polymers [35-37]. Various signal determination methods can be applied in the design of MIP-based sensors, but mostly potentiodynamic electrochemical techniques [34] or QCM-based [26,38] approaches are used for this purpose.
Development and application of MIPs in sensor design is reasonable because MIPs can be developed for small and low molecular weight molecules [12,39]. The efficiency of MIPs for the determination of some virus proteins was also demonstrated [34] and this technology recently was applied for the development of a molecularly imprinted poly-m-phenylenediamine based electrochemical sensors for the detection of SARS-CoV-2 proteins, namely, N-protein [40]. The sensor represented a disposable MIP-modified thin film electrode possessing selectivity to N-protein. Electrochemical signal was observed by DPV and a linear response to N-protein was up to 111 fM with a detection and quantification limit of 15 fM and 50 fM [40].
It should be noted that even some short DNA-based oligomers can be determined by MIP-based sensors [31], which makes MIP-based sensors attractive for DNA and probably for RNA fragment determination. Due to the rather low price of MIPs in comparison to that of antibodies, MIP-related research area is of particular interest and, therefore, MIPs potentially can replace antibodies during the design of various bioanalytical systems and immunosensors.
2.3. Ellipsometry and SPR Based Immunosensors
Optical ellipsometry-based techniques have great potential to be applied in the design of various immunosensors [41].
Recently, spectroscopic ellipsometry (SE) in total internal reflection mode (TIRE) was applied for the monitoring the kinetic of interactions between on SAM-modified gold disk immobilized SARS-CoV-2 N-protein and antibodies against it [42]. TIRE allowed detecting biomolecules mass changes at solid-liquid interface by phase shift measurement. The high sensitivity of SE TIRE was attained with the support of SPR, what enabled the registration of two kinetic curves Ψ(t) and Δ(t) simultaneously [43,44]. It was reported, that antigen-antibody complex is strongly bound and the complex formation has very strict orientation requirements, which was established by meaning of mathematical model building [42]. The main working element of the sensor is the piezoelectric resonator, on which an antigen or antibody is immobilized using SAM-based technology. Incidentally, the application of antibody fragments seems to be a very promising approach for the development of sensors for the determination of virus proteins because it enables increasing the surface concertation of sites that are selective to virus proteins [45,46].
2.4. Photoluminescence-Based Immunosensors
Photoluminescence is a very sensitive technique that can be applied in the design of various affinity biosensors for the determination of pathological cells [47] and virus-induced diseases [48-50]. Some researchers designed a split luciferase (spLUC) based antibody test that is showing itself as simple (not need ‘washing’, two-stage of reagent addition, rapid (less than 5 min), reliable (≥98%), low-volume specimen (1 µL for 1 reaction), inexpensive and solution-based quantitative approach to identify antibodies against S- and N- proteins of SARS-CoV-2 [51]. The biosensor was designed by merging small BiT (SmBiT) and large BiT (LgBiT) fragments [52] of Nanoluciferase (NanoLuc) to viral protein antigens. The immunoglobulin has two antigen-binding sites, thus the outcome of incubating 1:1 mixture of SmBiT and LgBiT with serum will be the coupling of one antigen-binding site with LgBiT and another site with SmBiT. The fixing of LgBiT and SmBiT fragments leads to the reduction of NanoLuc enzyme for the following luminescence-based identification (Figure 3).
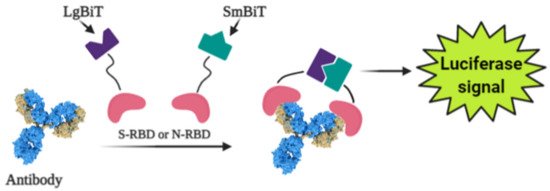
Figure 3. Scheme of the general working principle of split luciferase based immunosensor [1].
Sensors based on S- and N-proteins of the SARS-CoV-2 were designed because SARS-CoV-2 infected patients contain antibodies, which are primarily addressed to S- and N-protein epitopes [53,54]. The sensor-based on genetically engineered S-protein containing merged RBD with NanoLuc fragments, whereas for the creation of N-protein-based sensor N-terminal sequence was utilized. The ordinary differential equation modeling was executed to describe the ratio between signal intensity and immunoglobulin concentration and it was shown that there was a linear correlation between the specific antibody concentration and luciferase signal. The sensor showed sensitivity of 89% towards S-protein and 98% towards N-protein [51].While the existing ELISA-based analysis possesses such disadvantages as laboriousness with numerous washing stages, which complicates point-of-care diagnostics and implementation in regions with limited analytical hardware and reagent sources, the spLUC approach has critical properties that are compliant with all these usages [46]. The reagents used for the spLUC assay were demonstrated to be quite stable to lyophilization for storage and simple transport and rapidly identify immunoglobulins straight from the clinical specimens. The kit containing common pipettes and a portable luminometer is enough for readily setting of the spLUC assay at any care centers despite the infrastructure. The modularity is another benefit of the assay, which can allow accommodating the test to the immune response against almost any infection with known antigens [51].
2.5. Determination of Reactive Oxygen Species
It was reported that coronaviruses induce mitochondrial reactive oxygen species (ROS) promotes viral replications in lung host cells [55]. The detector of reactive oxygen species stimulated by COVID-19 is an electrochemical ROS/H2O2 system [56]. This device includes an integrated portable automatic real-time electrochemical readout board and a sensor, which was made from the multiwall carbon nanotube (MWCNT) on the tip of steel needles. The basic operating principle is the immersion of the electrode into sputum and the latching signals of reactive oxygen species. The intensity of ROS levels, which were released from viral-infected epithelium, were determined by CV.Unlike other ROS detection approaches, the electrochemical method is rapid (less than 30 s) and can be performed in vivo, without any additional specimen preparation. Electrochemical ROS detection assay was shown as the system operating with lower than 500 μL volumes of aliquots with a rather high accuracy of over 97% [57].
This entry is adapted from the peer-reviewed paper 10.3390/mi12040390
