The consumption of whole grain products is often related to beneficial effects on consumer health. Dietary fibre is an important component present in whole grains and is believed to be (at least partially) responsible for these health benefits. The dietary fibre composition of whole grains is very distinct over different grains. Whole grains of cereals and pseudo-cereals are rich in both soluble and insoluble functional dietary fibre that can be largely classified as e.g., cellulose, arabinoxylan, β-glucan, xyloglucan and fructan. However, even though the health benefits associated with the consumption of dietary fibre are well known to scientists, producers and consumers, the consumption of dietary fibre and whole grains around the world is substantially lower than the recommended levels. This review will discuss the types of dietary fibre commonly found in cereals and pseudo-cereals, their nutritional significance and health benefits observed in animal and human studies.
- dietary fibre
- cereals
- pseudo-cereals
- chronic diseases
1. Structure of Cereal Grains
Wheat, barley, oats and rye belong, as all true cereals do per definition, to the grass family (Poaceae). This family is a very diverse family, covering plants that humans have used to grow a lawn to plants that can grow several meters tall (bamboo) [1]. Cereal grains have a complex structure that is characterized by different cell layers. Although the individual structural parts of the different cereal kernels may differ significantly in terms of composition and size, the general cereal structure remains largely the same [2]. Three main parts can be distinguished: the embryo or germ, the endosperm, and the outer kernel layers that cover the embryo and endosperm or the so-called bran [3]. The starchy endosperm accounts for 80–85% of the grain. It is mainly composed of starch and protein. Bran and germ represent 12–18% and 2–3% of the dry grain weight, respectively [4]. The embryo is vital for the germination process, as it comprises the embryonic axis and scutellum. The embryo has the highest content of lipids and lipid-soluble vitamins of all fractions in the cereal kernel [5]. The endosperm has the highest economic importance. In the endosperm insoluble nutrients, mainly starch and proteins, are deposited as an energy source for the developing plant upon germination [4]. The aleurone layer is the outermost layer of the endosperm. It usually consists of 1 to 3 layers of cells, depending on the cereal. In some cases, pigmentation in the aleurone layer can give cereal kernels a distinct colour [5]. Although botanically, the aleurone layer is considered to be part of the endosperm, a major part of this layer has been shown to be removed during roller milling and, hence, is often not part of the refined white cereal flour [6]. The bran fraction consists of several different layers that can be distinguished from one another, i.e., outer pericarp, inner pericarp, testa, and nucellar epidermis (also called hyaline layer) (Figure 1) [4]. Inner and outer pericarp are rich in highly crosslinked polysaccharides, such as cellulose, lignin and heteroxylan [7][8]. The testa of barley, oats and rice is present as one cell layer, while wheat and rye testas generally have two distinct layers [3]. The nucellar epidermis is the maternal tissue that covers the endosperm, but is not prominent in all cereals [5]. In sorghum, this layer is very prominently present, but it is usually absent or only present as a thin layer in most other cereals [9].
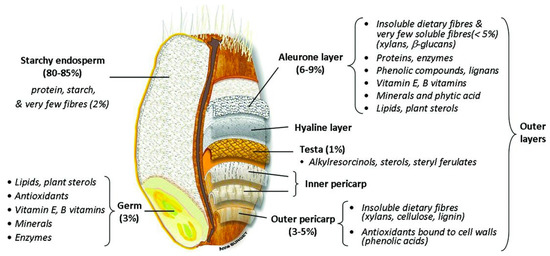
Figure 1. Wheat grain structure [10].
2. Dietary Fibre Composition of Different Cereals
Dietary fibres are defined as “carbohydrates with a degree of polymerization of 3 or more that naturally occur in foods of plant origin and that are not digested and absorbed by the small intestine” [11]. Dietary fibre can be classified according to its water solubility in insoluble dietary fibre (IDF) and soluble dietary fibre (SDF) [12]. IDF includes cellulose, water-insoluble hemicellulose and lignin, and are mainly present in plants as structural cell wall components [13]. SDF consists of a variety of non-cellulosic polysaccharides and oligosaccharides. Examples are pectins, β-glucans and water-soluble gums [14]. SDF and IDF differ largely in their functionality as food ingredients and their physiological effects upon consumption [12]. With regard to the latter, SDF, by increasing the viscosity of stomach and intestinal contents, is believed to reduce the overall intestinal enzymatic activity, and to decrease post-prandial plasma glucose levels [15][16]. In addition, SDF are highly fermentable and increase the production of short chain fatty acids (SCFAs), which are important contributors in the management of CVDs [17]. IDF, conversely, mainly serves as a bulking agent and laxative, hence, increasing faecal mass and decreasing intestinal transit time [18]. A potential mechanism of IDF related to management of NCDs might be related to increased satiety and reduction in body weight [17]. Both SDF and IDF help prevent constipation, decrease re-adsorption of bile salts, and lower the risk of colon cancer [14].
Dietary fibre can be obtained from different dietary sources, which include grains, fruits and vegetables. The amount and composition of dietary fibre can vary with the source [12]. Cereals are an important source of dietary fibre, contributing to about 50% of the total dietary fibre intake in Western countries [19]. Vegetables deliver about 30 to 49% of the daily dietary fibre intake, while fruits contributed about 16%. Equal weights of fruits and vegetables contain less total dietary fibre relative to cereal grains, due to the higher moisture content. The proportion of IDF of the total dietary fibre varies depending on the type of fruit or vegetable that is studied [12]. Cellulose is the main component in the IDF fraction in plants, while pectin is a major fraction in the SDF fraction of fruits and vegetables [20].
2.1. Dietary Fibre Composition of Wheat
The total dietary fibre content of wheat ranges from 9 to about 20% (dry weight basis), and is composed of both insoluble and soluble fractions (Table 1) [21][22]. The cell walls of the starchy endosperm cells in wheat are composed of two major types of dietary fibre components; i.e., arabinoxylan (AX) and β-d-glucan. These cell walls may also contain small amounts of cellulose and glucomannans [1]. The cellulose content in wheat endosperm is usually very low (<5%) [21]. Cellulose is a linear polymer of β-(1-4) linked glucose units, that associates with other cellulose molecules to form a highly insoluble network [22].
Table 1. Dietary fibre content (total, insoluble and soluble) of cereals and pseudo-cereals (g/100 g).
| TDF | IDF | SDF | Reference | |
|---|---|---|---|---|
| Wheat (Triticum aestivum L., Triticum durum Desf.) | 11.6–17.0 | 10.2–14.7 | 1.4–2.3 | [23] |
| 10.2–15.7 | 7.2–11.4 | 1.9–2.9 | [24] | |
| 9.2 | - | - | [25] | |
| Oat (Avena sativa L.) | 13.7–30.1 | - | 11.5–20.0 | [26] |
| 10.3 | 6.5 | 3.8 | [12] | |
| 11.5–37.7 | 8.6–33.9 | 2.9–3.8 | [27] | |
| Barley (Hordeum vulgare L.) | 14.6–27.1 | 12.0–22.1 | 2.6–5.0 | [27] |
| 16.8–27.9 | - | - | [28] | |
| 10.1 | - | - | [29] | |
| Rye (Secalecereale L.) | 15.2–20.9 | 11.1–15.9 | 3.7–4.5 | [21] |
| 14.7–20.9 | 10.8–15.9 | 3.4–6.6 | [30] | |
| Rice (Oryza sativa L.) | 9.9 | 5.4 | 4.4 | [31] |
| 2.7–4.9 | 1.9–4.2 | 0.6–1.1 | [32] | |
| Corn (Zea mays L.) | 3.7–8.6 | 3.1–6.1 | 0.5–2.5 | [33] |
| 13.1–19.6 | 11.6–16.0 | 1.5–3.6 | [27] | |
| Amaranth (Amaranthus spp.) | 8.9–20.6 | - | - | [34] |
| 11.4 | 7.7 | 3.7 | [35] | |
| 11.8 | 9.1 | 2.7 | [36] | |
| Quinoa (Chenopodium quinoa Willd.) | 7–9.5 | 4.9–5.6 | 2.1–3.9 | [35] |
| 16.2–21.6 | - | - | [37] | |
| 11.6–15.1 | 9.9–12.2 | 0.4–2.9 | [38] | |
| Buckwheat (Fagopyrumesculentum Moench.) | 7.0 | 2.2 | 4.8 | [39] |
| 11.9 | 5.8 | 6.1 | [40] | |
| Teff [Eragrostis tef (Zucc.) Trotter] | 4.54 | - | 0.85 | [41] |
| Sorghum (Sorghum bicolor) | 7.55–12.3 | 6.52–7.90 | 1.05–1.23 | [42] |
| Millets (Eleusine coracana (L.) Gaertn.) | 13.0–13.8 | 12.5–13.2 | 0.52–0.59 | [43] |
Hemicellulose is a prominent type of DF in grains. Hemicellulose is defined as the non-cellulosic component in cell walls consisting of heterogenic polysaccharides [44]. Hemicellulose molecules can be grouped largely into four categories: xylans, xyloglucans, glucomannans and mixed linkage β-glucans [44]. Hemicelluloses can be soluble or insoluble, depending upon their size and structure (e.g., side chain substitutions and intermolecular crosslinks) [45].
AX and mixed linked β-glucan account for about 70% and 20% of the total dietary fibre content, respectively. AX molecules are composed of a linear backbone of d-xylopyranosyl (Xyl) residues linked through β-(1-4) glycosidic linkages (Figure 2). Residues of α-L-arabinofuranosyl (Ara) can be attached to the Xyl residues at the O-2 and O-3 positions (Figure 2). Four structural elements can, hence, be found in AX: non-substituted, O-2 or O-3 monosubstituted and disubstituted Xyl [46]. Ferulic acid can be esterified to arabinose residues on the O-5 position [47]. These ferulic acid structures can form bridges between AX chains, resulting in an increase of the AX molecular weight and a decrease in its water-extractability.
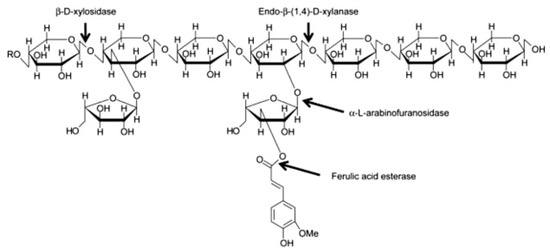
Figure 2. Structure of arabinoxylan [48].
A significant part of the AX (>30%) in wheat endosperm is present as a water-extractable (WE) fraction [49][50]. The water-unextractable AX (WU-AX) are typically crosslinked to other polysaccharides or lignin molecules in cell walls [51]. The structure of WU-AX is very similar to that of WE-AX, but the average molecular weight and (to some extent) the Ara/Xyl ratio are higher for WU-AX than for WE-AX [50] β-d-glucan has a relatively simple structure in cereals, as it is only built up of one monosaccharide, i.e., β-d-glucose, that can be linked through either β-1-4 or β-1-3 linkages [2].
Aleurone cells in wheat are characterized by thick cell walls. Relative to the starchy endosperm cell walls, the relative levels of AX and β-d-glucan in aleurone cell walls, however, remain the same [52]. AX in the aleurone layer, however, is highly esterified and crosslinked through diferulic acid bridges compared to starchy endosperm AX [53]. The pericarp cell wall composition is similar to the cell wall composition found in straw, characterized by highly branched AX. The AX in the pericarp also contain galactose and glucuronic acid residues and have a higher content of ferulic and diferulic acid residues [54].
2.2. Dietary Fibre Composition of Barley and Oats
Barley is one of the earliest cultivated cereals and exists in hulled and hulless varieties. Hulled barley can be dehulled after harvest prior to processing [55]. However, the hull from barley is not that easy to remove, as it is ‘cemented’ to the outer layer of the kernel or caryopsis, i.e., the pericarp [28]. In both hulled and hulless types, the caryopsis is composed of the pericarp, testa (seed coat), aleurone layer, endosperm, and embryo [55]. Oats, on the other hand, is also a hulled cereal, but its hull is relatively easy to remove. Barley and oats are an excellent source of soluble and insoluble dietary fibre and other bioactive compounds. Soluble dietary fibre (mainly β-glucan) is located in the endosperm cell walls, while the (predominantly) insoluble dietary fibre fraction (cellulose, AX and lignin) is mainly found in the cereal bran [56]. The total dietary fibre content of dehulled barley and oats ranges from 10 to 28% [28][29] and 10 to 38% [12][26][27] (on dry matter basis), respectively (Table 1). Both barley and oats contain β-glucan as the primary non-starch polysaccharide in the whole kernel. AX is also found in both cereals, but in a much lesser content. β-glucan and AX are typically present as 70 to 20% of the total dietary fibre content in these cereals. Cereal β-glucan is composed of cellotriosyl and cellotetraosyl units linked through β-1-3 linkages (Figure 3) [55]. The presence of such β-1-3 linkages leads to bends in the polymer chain structure, allowing water to get in between the chains [55]. This explains the higher solubility of β-glucan as compared to cellulose, a structurally related polymer built exclusively of β-1-4-linked d-glucose units [57].
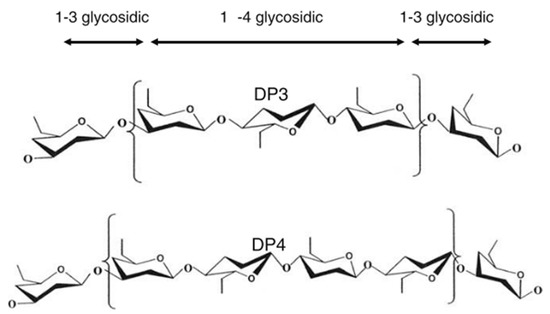
Figure 3. Molecular structure of cereal β-glucan [58].
The β-glucan content in oats and barley varies with the genotype. β-glucan is distributed uniformly throughout the endosperm in barley, while it is more concentrated in the outer layers of oats endosperm [58]. Whole grain barley can provide similar amounts of β-glucan as oats do. Hulless barley varieties and barley varieties with low amylose content can even provide 1.5 to 4 times more β-glucan as compared to oats [55].
As endosperm cell walls of barley and oats are rich in β-glucan, the β-glucan content of barley and oats may not decrease with the removal of the outer bran layers [55]. The soluble β-glucan content even increases in the function of dehulling, indicating the dominant endosperm distribution of β-glucan [59].
2.3. Dietary Fibre Composition of Rye
The dietary fibre content of rye is higher compared to wheat, as rye contains about 14 to 21% dietary fibre (Table 1) on dry matter base [27][60]. AX, cellulose, fructan and β-glucan are the dominant dietary fibre types in rye, with AX being the major dietary fibre component (i.e., 45% of total dietary fibre content) present in endosperm cell walls [60]. Although both rye and wheat contain AX, the content and solubility of AX in rye is higher compared to AX found in wheat [44].
Rye contains the highest amount of fructan among the here-discussed cereals. Fructan is a soluble dietary fibre composed of β-d-fructofuranosyl units, with or without terminal glucose residue [61]. Fructans of rye can be linear or branched in structure. A typical degree of polymerization of fructan in rye ranges anywhere from 2 to 60 [62].
The level of dietary fibre present in rye varies in function of its location within the kernel. The inner endosperm contains less dietary fibre (12%), while the outer endosperm and bran fraction contain about 22 and 38% dietary fibre on dry matter basis, respectively [63]. The higher levels of dietary fibre found in the outer kernel layers of rye are another illustration of the importance of eating whole grains.
2.4. Dietary Fibre Composition of Other Grains
The dietary fibre content of rice (whole grain) varies from 2.7 to 9.9% (Table 1). This high variation in dietary fibre content is partially related to differences found in between rice varieties [44][32]. The dietary fibre content of brown rice is higher than the content found in white rice, in which, essentially, the outer kernel layers have been removed by abrasive milling. As is the case with the other cereals, the dietary fibre is also mainly found in the hull and bran of rice kernels [64]. In rice (whole grain), the major components of the IDF fraction are cellulose and water insoluble hemicellulose, while soluble AX and β-glucan make up the SDF fraction [65].
The dietary fibre content of corn varies between 3.7 and 19.9% on dry matter basis [27][66], of which IDF is the largest fraction (Table 1) [23]. Cellulose and hemicellulose are the main IDF fractions found in corn bran [66].
3. Structure of Pseudocereal Grains
Pseudocereals are largely underutilized crops that have recently been gaining attention due to the nutritional properties that are associated with them. Pseudocereals can be processed into, and used as, a flour, in a very similar way to the way in which wheat is processed and used. The three pseudocereals that have been most widely studied thus far are amaranth (Amaranthus spp; Amaranthceae), quinoa (Chenopodium quinoa subsp. quinoa; Chenopodiaceae), and buckwheat (Fagopyrum esculentum; Polygonaceae) [40]. These are dicotyledonous plants, as opposed to cereals like wheat, rice and barley, which are monocotyledonous [67].
Pseudocereal seeds are, similarly to cereals, also composed of several ‘layers’ (Figure 4). Perisperm, germ and endosperm are the three main areas containing food reserves in pseudocereals [66]. The kernel and ‘layer’ composition varies for the different pseudocereals.
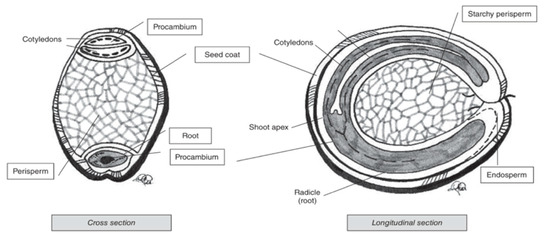
Figure 4. Cross and longitudinal section of amaranth seed [66].
Amaranth is a small seed with diameters ranging from around 0.9 to 1.7 mm [66]. The major portion of the seed is the embryo, which is twisted in a circle. The embryo is large and encloses the perisperm and consists of the radicle and cotyledons, which is the main protein storage organ of the seed [68]. The seed coat is completely smooth and thin, and its colour can be white, cream, gold-yellow and even brown [40]. Quinoa kernels have the same structure as amaranth kernels. It produces small, spherical-shaped seeds, with diameters that vary between 1.0 and 2.6 mm. One gram of quinoa contains about 250 to 500 seeds [69]. Similar to what is the case for amaranth, the main storage tissues of quinoa seeds are the perisperm, embryo and cotyledons [70]. Buckwheat seed is pyramid-shaped with sizes ranging from 4 to 9 mm [69]. The seed is covered with a dull brown or grey pericarp that is tightly attached to the seed [71]. The embryo is embedded in the centre of the endosperm and has two cotyledons [72].
4. Dietary Fibre Composition of Pseudocereals
Amaranth, quinoa and buckwheat are pseudocereals with a long history of utilization as food ingredients and have very interesting nutritional characteristics. Pseudocereals have been gaining popularity in the last decade as ingredient for gluten-free products. Their use substantially increases the dietary fibre content of these products, which are typically deficient in dietary fibre [34]. Although the dietary fibre content varies between amaranth species, the total dietary fibre content of amaranth varies between 9 and 21% (dry weight basis) [73][74], while quinoa contains around 7 to 21% TDF [35][37][38]. Based on a monosaccharide analysis of dietary fibre extracted from amaranth and quinoa samples, the dietary fibre in these pseudocereals is mainly composed of galacturonic acid, arabinose, xylose, glucose and galactose. According to this monosaccharide composition and analysis of the linkages, the dominant fraction of both the soluble and insoluble dietary fibre in these pseudocereals is classified as pectic polysaccharide [75]. Xyloglucans are the second most prominent dietary fibre present in amaranth and quinoa whole grains. Cell walls of amaranth also contain significant amounts of phenolic acids. High levels of ferulic acid were found, while coumaric acid and caffeic acid are also present, but in lower amounts [68]. Both quinoa and amaranth have a high proportion (~22% of the total dietary fibre content) of SDF compared to wheat (about 15%), indicating promising potential with regard to colon health [76].
The total dietary fibre content of buckwheat groats (7–11.9%) is lower than the dietary fibre content found in most cereals such as wheat, barley and oats (Table 1). The majority (~70%) of dietary fibre from buckwheat groats is water insoluble [72]. The water soluble fibre of buckwheat seeds is mostly classified as pectin, arabinogalactan and xyloglucan [71]. Pectin was found in the cell walls of the outer and inner epidermis, and the endosperm of buckwheat seeds [71].
This entry is adapted from the peer-reviewed paper 10.3390/nu12103045
References
- Evers, A.D.; Blakeney, A.B.; O’Brien, L. Cereal structure and composition. Aust. J. Agric. Res. 1999, 50, 629–650.
- Delcour, J.A.; Poutanen, K. Fibre-Rich and Wholegrain Foods: Improving Quality; Woodhead Publishing: Cambridge, UK, 2013.
- Liu, K. Physical properties of DDGS. In Distillers Grains: Production, Properties, and Utilization; Liu, K., Kurt, A.R., Eds.; AOCS Publishing: Urbana, IL, USA, 2016; pp. 121–142.
- Brouns, F.; Hemery, Y.; Price, R.; Anson, N.M. Wheat Aleurone: Separation, Composition, Health Aspects, and Potential Food Use. Crit. Rev. Food Sci. Nutr. 2012, 52, 553–568.
- Evers, T.; Millar, S. Cereal grain structure and development: Some implications for quality. J. Cereal Sci. 2002, 36, 261–284.
- Dexter, J.E.; Wood, P.J. Recent applications of debranning of wheat before milling. Trends Food Sci. Technol. 1996, 7, 35–41.
- Barron, C.; Surget, A.; Rouau, X. Relative amounts of tissues in mature wheat (Triticum aestivum L.) grain and their carbohydrate and phenolic acid composition. J. Cereal Sci. 2007, 45, 88–96.
- Rosa-Sibakov, N.; Poutanen, K.; Micard, V. How does wheat grain, bran and aleurone structure impact their nutritional and technological properties? Trends Food Sci. Technol. 2015, 41, 118–134.
- Liu, K. Structure and Grain Composition. In Distillers Grains: Production, Properties, and Utilization; Liu, K., Rosentrater, K.A., Eds.; AOCS Publishing: Urbana, IL, USA, 2016; pp. 45–69.
- Surget, A.; Barron, C. Histologie du grain de bl´e. Ind. des C´er´eales 2005, 145, 3–7.
- Health Canada. List of Dietary Fibres Reviewed and Accepted by Health Canada’s Food Directorate—Canada.ca. Available online: (accessed on 12 August 2020).
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266.
- Li, Y.O.; Komarek, A.R. Dietary fibre basics: Health, nutrition, analysis, and applications. Food Qual. Saf. 2017, 1, 47–59.
- Dai, F.J.; Chau, C.F. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017, 25, 37–42.
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. Beta glucan: A valuable functional ingredient in foods. Crit. Rev. Food Sci. Nutr. 2012, 52.
- Wood, P.J. Cereal β-glucans in diet and health. J. Cereal Sci. 2007, 46, 230–238.
- Weickert, M.O.; Pfeiffer, A.F. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J. Nutr. 2018, 148, 7–12.
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289.
- Cooke, R. ProQuest Ebook Central. Charlest. Advis. 2017, 19, 39–43.
- Esteban, R.M.; Mollá, E.; Benítez, V. Sources of Fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease: Fiber’s Interaction between Gut Micoflora, Sugar Metabolism, Weight Control and Cardiovascular Health; Academic Press: London, UK, 2017; pp. 121–146.
- Gartaula, G.; Dhital, S.; Netzel, G.; Flanagan, B.M.; Yakubov, G.E.; Beahan, C.T.; Collins, H.M.; Burton, R.A.; Bacic, A.; Gidley, M.J. Quantitative structural organisation model for wheat endosperm cell walls: Cellulose as an important constituent. Carbohydr. Polym. 2018, 196, 199–208.
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81.
- De Santis, M.A.; Kosik, O.; Passmore, D.; Flagella, Z.; Shewry, P.R.; Lovegrove, A. Comparison of the dietary fibre composition of old and modern durum wheat (Triticum turgidum spp. durum) genotypes. Food Chem. 2018, 244, 304–310.
- Rainakari, A.-I.; Rita, H.; Putkonen, T.; Pastell, H. New dietary fibre content results for cereals in the Nordic countries using AOAC 2011.25 method. J. Food Compos. Anal. 2016, 51, 1–8.
- Dodevska, M.S.; Djordjevic, B.I.; Sobajic, S.S.; Miletic, I.D.; Djordjevic, P.B.; Dimitrijevic-Sreckovic, V.S. Characterisation of dietary fibre components in cereals and legumes used in Serbian diet. Food Chem. 2013, 141, 1624–1629.
- Šterna, V.; Zute, S.; Jansone, I.; Kantane, I. Chemical Composition of Covered and Naked Spring Barley Varieties and Their Potential for Food Production. Polish J. Food Nutr. Sci. 2017, 67, 151–158.
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463.
- Djurle, S.; Andersson, A.A.M.; Andersson, R. Milling and extrusion of six barley varieties, effects on dietary fibre and starch content and composition. J. Cereal Sci. 2016, 72, 146–152.
- Messia, M.C.; Candigliota, T.; De Arcangelis, E.; Marconi, E. Arabinoxylans and β-glucans assessment in cereals. Ital. J. Food Sci. 2017, 29, 112–122.
- Hansen, H.B.; Rasmussen, C.V.; Bach Knudsen, K.E.; Hansen, A. Effects of genotype and harvest year on content and composition of dietary fibre in rye (Secale cereale L.) grain. J. Sci. Food Agric. 2003, 83, 76–85.
- Amalraj, A.; Pius, A. Influence of oxalate, phytate, tannin, dietary fiber, and cooking on calcium bioavailability of commonly consumed cereals and millets in India. Cereal Chem. 2015, 92, 389–394.
- Prasad, V.S.S.; Hymavathi, A.; Babu, V.R.; Longvah, T. Nutritional composition in relation to glycemic potential of popular Indian rice varieties. Food Chem. 2018, 238, 29–34.
- Prasanthi, P.S.; Naveena, N.; Vishnuvardhana Rao, M.; Bhaskarachary, K. Compositional variability of nutrients and phytochemicals in corn after processing. J. Food Sci. Technol. 2017, 54, 1080–1090.
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113.
- Srichuwong, S.; Curti, D.; Austin, S.; King, R.; Lamothe, L.; Gloria-Hernandez, H. Physicochemical properties and starch digestibility of whole grain sorghums, millet, quinoa and amaranth flours, as affected by starch and non-starch constituents. Food Chem. 2017, 233, 1–10.
- Robin, F.; Théoduloz, C.; Srichuwong, S. Properties of extruded whole grain cereals and pseudocereals flours. Int. J. Food Sci. Technol. 2015, 50, 2152–2159.
- Pulvento, C.; Riccardi, M.; Lavini, A.; Iafelice, G.; Marconi, E.; D’Andria, R. Yield and Quality Characteristics of Quinoa Grown in Open Field Under Different Saline and Non-Saline Irrigation Regimes. J. Agron. Crop Sci. 2012, 198, 254–263.
- Miranda, M.; Vega-Gálvez, A.; Martínez, E.A.; López, J.; Marín, R.; Aranda, M.; Fuentes, F. Influence of contrasting environments on seed composition of two quinoa genotypes: Nutritional and functional properties. Chil. J. Agric. Res. 2013, 73, 6–7.
- Steadman, K.J.; Burgoon, M.S.; Lewis, B.A.; Edwardson, S.E.; Obendorf, R.L. Buckwheat seed milling fractions: Description, macronutrient composition and dietary fibre. J. Cereal Sci. 2001, 33, 271–278.
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional constituents of pseudo cereals and their potential use in food systems: A review. Trends Food Sci. Technol. 2018, 75, 170–180.
- Zhu, F. Chemical composition and food uses of teff (Eragrostis tef). Food Chem. 2018, 239, 402–415.
- Bach Knudsen, K.E.; Munck, L. Dietary fibre contents and compositions of sorghum and sorghum-based foods. J. Cereal Sci. 1985, 3, 153–164.
- Jayawardana, S.A.S.; Samarasekera, J.K.R.R.; Hettiarachchi, G.H.C.M.; Gooneratne, J.; Mazumdar, S.D.; Banerjee, R. Dietary fibers, starch fractions and nutritional composition of finger millet varieties cultivated in Sri Lanka. J. Food Compos. Anal. 2019, 82, 103249.
- Ciudad-Mulero, M.; Fernández-Ruiz, V.; Matallana-González, M.C.; Morales, P. Dietary fiber sources and human benefits: The case study of cereal and pseudocereals. Adv. Food Nutr. Res. 2019, 90, 83–134.
- Sharma, P.; Bhandari, C.; Kumar, S.; Sharma, B.; Bhadwal, P.; Agnihotri, N. Dietary Fibers: A Way to a Healthy Microbiome. Diet Microbiome Health 2018, 299–345.
- Izydorczyk, M.S.; Dexter, J.E. Barley b-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products—A Review. Food Res. Int. 2008, 41, 850–868.
- Aguedo, M.; Fougnies, C.; Dermience, M.; Richel, A. Extraction by three processes of arabinoxylans from wheat bran and characterization of the fractions obtained. Carbohydr. Polym. 2014, 105, 317–324.
- Dornez, E.; Gebruers, K.; Delcour, J.A.; Courtin, C.M. Grain-associated xylanases: Occurrence, variability, and implications for cereal processing. Trends Food Sci. Technol. 2009, 20, 495–510.
- Marcotuli, I.; Hsieh, Y.S.Y.; Lahnstein, J.; Yap, K.; Burton, R.A.; Blanco, A.; Fincher, G.B.; Gadaleta, A. Structural Variation and Content of Arabinoxylans in Endosperm and Bran of Durum Wheat (Triticum turgidum L.). J. Agric. Food Chem. 2016, 64, 2883–2892.
- Michelini, E.; Guardigli, M.; Magliulo, M.; Mirasoli, M.; Roda, A.; Simoni, P.; Baraldini, M. Bioluminescent biosensors based on genetically engineered living cells in environmental and food analysis. Anal. Lett. 2006, 39, 1503–1515.
- Delcour, J.A.; Van Win, H.; Grobet, P.J. Distribution and structural variation of arabinoxylans in common wheat mill streams. J. Agric. Food Chem. 1999, 47, 271–275.
- Parker, M.L.; Ng, A.; Waldron, K.W. The phenolic acid and polysaccharide composition of cell walls of bran layers of mature wheat (Triticum aestivum L. cv. Avalon) grains. J. Sci. Food Agric. 2005, 85, 2539–2547.
- Saulnier, L.; Sado, P.E.; Branlard, G.; Charmet, G.; Guillon, F. Wheat arabinoxylans: Exploiting variation in amount and composition to develop enhanced varieties. J. Cereal Sci. 2007, 46, 261–281.
- Barron, C.; Bar-L’Helgouac’h, C.; Champ, M.; Saulnier, L. Arabinoxylan content and grain tissue distribution are good predictors of the dietary fibre content and their nutritional properties in wheat products. Food Chem. 2020, 328, 8.
- Newman, C.W.; Newman, R.K.; Fastnaught, C.E. Barley. In Whole Grains and Their Bioactives: Composition and Health; Johnson, J., Wallace, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 135–167.
- Andersson, A.A.M.; Lampi, A.M.; Nyström, L.; Piironen, V.; Li, L.; Ward, J.L.; Gebruers, K.; Courtin, C.M.; Delcour, J.A.; Boros, D.; et al. Phytochemical and dietary fiber components in barley varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9767–9776.
- Lazaridou, A.; Biliaderis, C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007, 46, 101–118.
- Vasanthan, T.; Temelli, F. Grain fractionation technologies for cereal beta-glucan concentration. Food Res. Int. 2008, 41, 876–881.
- Panfili, G.; Fratianni, A.; Di Criscio, T.; Marconi, E. Tocol and β-glucan levels in barley varieties and in pearling by-products. Food Chem. 2008, 107, 84–91.
- Andersson, R.; Fransson, G.; Tietjen, M.; Åman, P. Content and molecular-weight distribution of dietary fiber components in whole-grain rye flour and bread. J. Agric. Food Chem. 2009, 57, 2004–2008.
- Meija, L.; Krams, I. Rye. In Whole Grains and Their Bioactives: Composition and Health; Johnson, J., Wallace, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 169–208.
- Andersson, A.A.M.; Dimberg, L.; Åman, P.; Landberg, R. Recent findings on certain bioactive components in whole grain wheat and rye. J. Cereal Sci. 2014, 59, 294–311.
- Rakha, A.; Åman, P.; Andersson, R. Characterisation of dietary fibre components in rye products. Food Chem. 2010, 119, 859–867.
- Ji, C.M.; Shin, J.A.; Cho, J.W.; Lee, K.T. Nutritional evaluation of immature grains in two Korean rice cultivars during maturation. Food Sci. Biotechnol. 2013, 22, 903–908.
- Fernando, B. Rice as a Source of Fibre. Rice Res. Open Access 2013, 1.
- Arendt, E.K.; Zannini, E. Cereal Grains for the Food and Beverage Industries; Woodhead Publishing: Cambridge, UK, 2013.
- Fletcher, R.J. Pseudocereals: Overview. In Encyclopedia of Food Grains, 2nd ed.; Academic Press: London, UK, 2013; pp. 488–493.
- Velarde-Salcedo, A.J.; Bojórquez-Velázquez, E.; de la Rosa, A.P.B. Amaranth. In Whole Grains and Their Bioactives: Composition and Health; Johnson, J., Wallace, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 209–250.
- Reguera, M.; Haros, C.M. Structure and Composition of Kernels. In Pseudocereals: Chemistry and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 28–48.
- Prego, I.; Maldonado, S.; Otegui, M. Seed structure and localization of reserves in Chenopodium quinoa. Ann. Bot. 1998, 82, 481–488.
- Izydorczyk, M.S.; McMillan, T.; Bazin, S.; Kletke, J.; Dushnicky, L.; Dexter, J. Canadian buckwheat: A unique, useful and under-utilized crop. Can. J. Plant Sci. 2014, 94, 509–524.
- Izydorczyk, M.S.; Head, D. Characterization and Potential Uses of Functional Buckwheat Fractions Obtained by Roller Milling of New Canadian Buckwheat Genotypes. Eur. J. Plant Sci. Biotechnol. 2010, 4, 71–81.
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int. J. Food Sci. Nutr. 2009, 60, 240–257.
- Mustafa, A.F.; Seguin, P.; Gélinas, B.; Gé Linas, B. Chemical composition, dietary fibre, tannins and minerals of grain amaranth genotypes. Int. J. Food Sci. Nutr. 2011, 62, 750–754.
- Lamothe, L.M.; Srichuwong, S.; Reuhs, B.L.; Hamaker, B.R. Quinoa (Chenopodium quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary fibres high in pectic substances and xyloglucans. Food Chem. 2015, 167, 490–496.
- Haros, C.M.; Schonlechner, R. Pseudocereals: Chemistry and Technology; Schonlechner, C.M.H.R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016.
