Angiogenesis plays an important role in several physiological and pathological processes. Pharmacological angiogenesis modulation has been robustly demonstrated to achieve clinical benefits in several cancers. Adrenocortical carcinomas (ACC) are rare tumors that often have a poor prognosis. In addition, therapeutic options for ACC are limited. Understanding the mechanisms that regulate adrenocortical angiogenesis along the embryonic development and in ACC could provide important clues on how these processes could be pharmacologically modulated for ACC treatment. In this report, we performed an integrative review on adrenal cortex angiogenesis regulation in physiological conditions and ACC. During embryonic development, adrenal angiogenesis is regulated by both VEGF and Ang-Tie signaling pathways. In ACC, early research efforts were focused on VEGF signaling and this pathway was identified as a good prognostic factor and thus a promising therapeutic target.
- angiogenesis
- adrenal fetal cortex
- adrenocortical carcinoma
1. Introduction
2. Angiogenesis Regulation
- (1)
-
Sprouting angiogenesis, one the most well characterized mechanism leading to angiogenesis, relies on endothelial cells function specification into either tip or stalk cells. Tip cells are derived from the parent vessel, degrade the basement membrane, extend large filopodia which can sense angiogenic factor gradients, such as vascular endothelial growth factor (VEGF), and migrate along the chemotactic paths. In contrast, stalk cells proliferate behind tip cells to form the sprout body, start the process of lumen formation, and connect with neighboring vessels [5,6,7].
- (2)
-
Intussusceptive angiogenesis is a process that consists in the splitting of pre-existing vessels into two new vessels. It starts with the formation of transluminal tissue pillars through the invagination of opposing capillary endothelial cells into the vascular lumen, creating a zone of contact. Commonly, intussusceptive and sprouting angiogenesis are complementary mechanisms [5,8].
- (3)
-
Recruitment of endothelial progenitor cells and vasculogenesis, a process through which endothelial progenitor cells are recruited in response to several growth factors, cytokines and/or hypoxia-inducible factors. Endothelial progenitor cells differentiate into mature endothelial cells and are incorporated into the angiogenic sprout, thus contributing to new blood vessel formation [4,9].
- (4)
2.1. VEGF Pathway in Angiogenesis Regulation
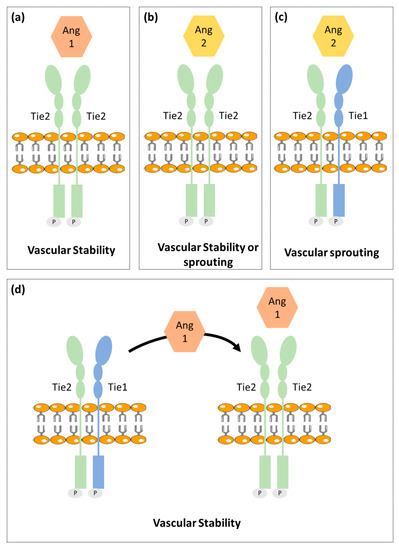
2.2. Ang-Tie Pathway in Angiogenesis Regulation
3. Angiogenesis in Normal Adrenal Cortex
3.1. Fetal Adrenal Cortex
3.2. Adult Adrenal Cortex
4. Angiogenesis in Adrenocortical Tumors
| Patient Group Comparisons | Results | |
|---|---|---|
| VEGF | Patients with ACT vs. Healthy individuals | ↑ VEGF serum levels in patients with ACT [59,60] |
| Aldosterone secreting ACA vs. Non-functioning ACA | ↑ VEGF tumor expression in aldosterone producing ACA [61] | |
| Cortisol secreting ACA vs. Aldosterone secreting ACA | ↑ VEGF serum levels patients with cortisol secreting ACA [60] | |
| ACC vs. Normal adrenal glands | ↑ VEGF expression in ACC [61,62] |
|
| ACC vs. ACA | ↑ VEGF serum levels in ACC ↑ VEGF tumor expression in ACC [59,61,63,64] |
|
| Patients with recurrent ACC vs. Patients with non-recurrent ACC | ↑ VEGF serum levels in recurrent ACC ↑ VEGF tumor expression in recurrent ACC [60,63] |
|
| Localized ACC vs. Invasive ACC | No difference in VEGF tumor expression [63] |
|
| VEGF-R2 | ACC vs. Normal adrenal glands | ↑ VEGF-R2 tumor expression in ACC [62] |
| ACC vs. ACA | ↑ VEGF-R2 tumor expression in ACC [64] |
This entry is adapted from the peer-reviewed paper 10.3390/cancers13051030
