Extracellular vesicles (EVs) are composed of a lipid bilayer containing transmembrane and soluble proteins. Subtypes of EVs include ectosomes (microparticles/microvesicles), exosomes, and apoptotic bodies that can be released by various tissues into biological fluids. EV cargo can modulate physiological and pathological processes in recipient cells through near- and long-distance intercellular communication. Recent studies have shown that origin, amount, and internal cargos (nucleic acids, proteins, and lipids) of EVs are variable under different pathological conditions, including cardiovascular diseases (CVD). The early detection and management of CVD reduce premature morbidity and mortality. Circulating EVs have attracted great interest as a potential biomarker for diagnostics and follow-up of CVD.
1. Introduction
Extracellular vesicles (EVs) is a generic term for particles naturally released from the cells that are delimited by a lipid bilayer-containing transmembrane and soluble proteins and cannot replicate, according to The International Society for Extracellular Vesicles (ISEV) [
1]. The first study that reported EVs was published in 1967 describing EVs as minute dust-like particulate material rich in lipid content [
2].
EVs can be classified based on size as small EVs (sEVs), with range < 100 nm or < 200 nm, and medium/large EVs (m/lEVs), with size range > 200 nm [
1]. EVs also can be classified based on cell origin as ectosomes (microparticles/microvesicles), exosomes, and apoptotic bodies. Ectosomes (size range 100–500 nm) are released from the plasma membrane budding, exosomes (size range 50–150 nm) are assembled from the endosomal pathway and released by exocytosis of multivesicular bodies (MVB), and apoptotic bodies (size range 500 nm–2 µm) are generated during apoptotic cell shrinkage and death [
3,
4,
5,
6]. There are various methods used for isolation of EVs or a specific EV subtype that have been recently reviewed [
7], such as ultracentrifugation (UC), size-exclusion chromatography (SEC), filtration, immunoaffinity-based isolation, commercial reagents (using polymers), microfluidics, and asymmetric flow field-flow fractionation (AF4). To increase the specificity or purity, the methods can be combined.
EVs can be characterized by their cargos and surface protein biomarkers, including annexins (e.g., annexin 1, 5, 6, and 11), disintegrin and metalloproteinase domain-containing protein 10 (ADAM10), angiotensin-converting enzyme (ACE), EH domain-containing protein 4 (EHD4), major histocompatibility complex class II (MHC II), flotillin-1 (FLOT1), and heat-shock 70-kDA (HSC70/HSP73, HSP70/HSP72). Other proteins are used as exosome markers, such as tetraspanins (CD9, CD63, CD81, and CD82), stress proteins (Hsc70 and Hsp90), proteins involved in membrane fusion (Rabs, and ARF6), and protein members of the endosomal sorting complex required for transport (Alix and TSG101) [
8,
9]. Microvesicles have content similar to exosomes that include specific proteins, such as integrins, glycoproteins, and metalloproteinases [
8,
10]. To identify EV’s protein markers, the main methods include Western blotting, ELISA, flow cytometry (FCM), and nano-FCM. In addition, transmission electron microscopy (TEM), dynamic light scattering (DLS), and nanoparticle tracking analysis (NTA) are commonly used [
7,
11,
12].
EVs have emerged as possible biomarker sources from several diseases, due their ability to modulate near- or in long-distance intercellular communication influencing the disease development and progression [
13,
14,
15]. Intercellular communication consists of transferring EV bioactive cargos or activating signaling pathways to recipient cells, which can lead to phenotypic and functional changes in their target cells [
5,
16]. EVs are present in various tissues and biological fluids from which they can be recovered and monitored in both physiological and pathological conditions [
17,
18]. The quantity, origin, and internal cargo (e.g., nucleic acids, proteins, and lipids from parental cells) are variable in different pathophysiological processes [
14,
19]. EVs also have a metabolically active outer membrane that protects their content until released into recipient cells [
17].
Circulating EVs have attracted great interest in the field of cardiovascular medicine due to their high stability. EVs offer a non-invasive access to monitor the status of the cardiovascular diseases (CVD), and the use of circulating EVs as diagnostic biomarkers [
13,
20]. CVD causes the highest number of deaths and vast health and economic burdens worldwide [
21,
22]. CVD include several pathologies such as coronary artery disease (CAD), cerebrovascular disease, peripheral arterial disease, ischemic heart disease, hypertension, and heart failure (HF). Early detection and management of CVD can decrease the risk of heart attack and stroke in individuals at high risk of CVD, and, therefore, reduce premature morbidity and mortality [
23].
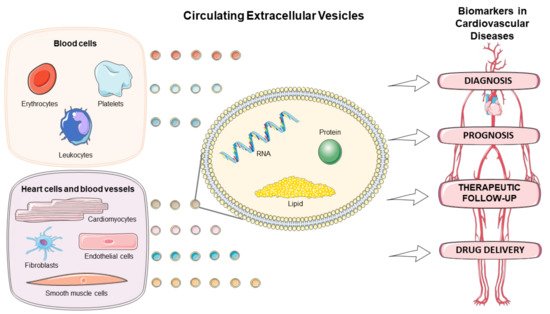
There has been a growing interest in exploring the EVs in the diagnostic, prognostic, and therapeutic monitoring of CVD, as well as drug delivery (). This review discusses the role of circulating EVs in CVD based on origin, amount, and content of the EVs, and highlights their application as biomarkers and drug delivery tool in several cardiovascular pathologies.
Figure 1. Circulating extracellular vesicles as biomarkers for diagnosis, prognosis, therapeutic follow-up, and drug delivery vehicles in cardiovascular diseases. Figure created using Servier Medical Art images (
http://smart.servier.com, accessed on 30 December 2020).
2. Extracellular Vesicles Quantification as Biomarker in CVD
Several studies have shown an association of circulating EV counts with CVD, suggesting a potential application of EV quantification as a biomarker for diagnostic and therapeutic monitoring [
25,
82,
83]. Although using EV counts from particular cell type as biomarker seems promising, the major limitation of this approach is the lack of standardization of methods, resulting in difficulty to compare studies from multiple research groups [
25].
The release of platelet-derived EVs was shown to be increased in plasma, under conditions with enhanced platelet activation, such as myocardial infarction and exposure to modified lipoproteins [
33,
84]. Likewise, in arterial and venous thrombosis, the activated platelets increase the circulating EV counts compared with healthy condition [
25].
Patients with atherothrombotic diseases and atherosclerotic lesions have high levels of circulating EVs derived from endothelial cells, vascular SMC, platelets, leukocytes or erythrocytes [
85]. Sansone et al. observed an increase of endothelial-derived EVs in the plasma of patients with arterial hypertension with and without CAD [
56]. Plasma levels of leukocyte-derived EVs were reported to be increased in atherosclerotic patients, and they were correlated with the progression of the atherosclerosis [
79].
Several studies have reported increased counts of EVs in ACS conditions [
86,
87,
88,
89,
90]. Serum EVs were found to be higher in patients with STEMI than whose with stable angina or control subjects, suggesting early stages increases in the disease due to thrombus formation and ischemia-induced stress [
91]. Erythrocyte-derived EV counts were also elevated in STEMI patients [
46].
Importantly, the increase of circulating EVs can be detected shortly after the pathological stimulus. Deddens et al. [
92] demonstrated that plasma EVs are rapidly detectable. In one study, the amount of EVs was already increased one hour after myocardial infarction. Ge et al. [
93] observed a significant increase in heart tissue EVs release 24 h after myocardial ischemia/reperfusion (I/R).
Patients with persistent atrial fibrillation (AF) and a high level of inflammation showed markedly increased EV concentration compared to subjects without AF [
82]. In addition, the inflammation contributes to platelet activation that induces the release of EVs in a prothrombotic state [
82]. A recent study also showed that circulating EVs were increased in patients with AF and a higher risk of stroke than non-AF patients of similar age [
94].
Circulating EVs derived from endothelial cells were explored in a prospective study, which demonstrated that patients with HF had increased plasma levels of endothelium-derived microparticles compared to healthy subjects [
95]. These HF patients had a higher probability of cardiovascular events (e.g., cardiovascular death, non-fatal myocardial infarction, ischemic stroke, or re-hospitalization related to HF), and it was suggested that EV counts could be a useful prognostic biomarker. Patients with symptoms of chronic HF had increased number of circulating endothelial-derived EVs that were correlated with increase of mortality and recurrent hospitalization risk due to HF [
96]. HF patients also had increased serum levels of EVs compared to healthy subjects [
97]. A recent review [
58] has reported that the number of EVs might be important to differentiate the severity of HF.
Circulating EV counts are also altered in patients with metabolic disorders that increase the risk of CVD. For example, the total number of circulating EVs was shown to be higher in patients with metabolic syndrome (MetS) compared to non-MetS subjects [
98]. Increased levels of endothelial-derived EVs were also observed in diabetic patients compared with healthy controls, and they were closely associated with vascular dysfunction [
99]. Circulating levels of lymphocyte-derived EVs were also increased in patients with familial hypercholesterolemia [
100].
3. Extracellular Vesicle as Biomarkers in CVD
Studies on the important regulatory effects of EVs in CVD has been motivated due to EV stability, their specific signatures associated with cell activation or injury, and their intrinsic activity and immunomodulatory properties [
13]. The changes in EV cargo, including RNAs, proteins, and lipids, as potential biomarkers in CVD are reviewed in .
Table 1. Summary of extracellular vesicles (EV) cargo as biomarkers in cardiovascular disease.
3.1. Extracellular Vesicles Carrying RNAs
The EV transcriptome of various cell types is important due to the biological relevance of RNA activity in several cardiovascular pathologies [
58,
109,
125,
126]. EVs are carriers of various RNA types, such as messenger RNA (mRNA), transfer RNA (tRNA), small interference RNA (siRNA), long-non-coding RNA (lncRNA), and microRNA (miRNA) [
13]. An earlier study identified mRNAs and miRNAs in EVs by microarray technology and showed the transference of functional RNA between three cell lines [
127].
Kenneweg et al. [
101] showed lncRNA-enriched EVs in cardiac ischemia. In this context, lncRNA
Neat1 was necessary for fibroblast and cardiomyocyte survival, and the silencing of
Neat1 resulted in reduced heart function after myocardial infarction. A study identified 185 differentially expressed circular RNAs (circRNAs), covalently closed RNAs, involved in the metabolic process from EVs of the murine heart post-I/R injury compared with control, and these circRNAs may regulate target genes by acting on the miRNAs [
93].
miRNAs are short non-coding RNAs (19-22 nucleotides) that regulate gene expression at the post-transcriptional level by binding to specific mRNAs with varying degrees of complementarity and leading to mRNA degradation and/or translational inhibition [
128,
129]. miRNAs control different physiological processes and abnormal patterns of expression have already been associated with many diseases [
129]. Different types of cells can release miRNAs into the extracellular space in response to various stimuli and pathologies [
130,
131]. In peripheral circulation, EVs are responsible for protecting miRNAs from degradation by circulating ribonucleases [
130,
132].
The EV-miRNAs can be promising predictors or indicators for premature CVD detection. Increased expression of miR-126 and miR-199a isolated from circulating EVs was proposed to reduce the risk of major cardiovascular outcomes in patients with CAD [
102]. Cheng et al. [
133] suggested that expression of miR-126 and miR-21 could be used for early detection of CVD, such as acute myocardial infarction and FH. Another study reported reduced plasma levels of EV-miR-126 in high-risk CVD patients and EV-miR-126 levels were negatively correlated with cardiac troponin I (cTnI) and N-terminal propeptide of B-type natriuretic peptide (NT-proBNP), suggesting miR-126 as a potential biomarker for CVD [
103].
miRNA-208a expression was upregulated in the serum exosomes of ACS patients, and the study suggested its important role for the early diagnosis and prognosis of ACS [
105]. Two EV-miRNA (miR-30e and miR-92a) that target ATP binding cassette (ABC)A1 were shown to be upregulated in plasma EVs from patients with coronary atherosclerosis [
104]. Endothelial cells-derived EVs containing miR-92a were increased in patients with CAD, and this miRNA was shown to regulate angiogenesis in recipient endothelial cells [
109]. EV-enriched miR-92a can be transferred from endothelial cells to macrophages and suppress Kruppel-like factor 4 (KLF4) expression in recipient cells, resulting in an atherosclerotic phenotype [
110,
134]. In addition, upregulation of the miR-1 in hepatocyte-derived EVs was associated with promotion of endothelial inflammation and facilitate atherogenesis by downregulation of KLF4 and activation of the NF-κB [
135].
Increased levels of urinary EVs miRNAs were reported in patients with unstable CAD compared to whose with stable CAD [
108]. The authors suggested an important role of miR-155 in disease progression that could be used as prognostic indicator and therapeutic target.
Atherogenic EVs from mouse and human macrophages were enriched in miR-146a, miR-128, miR-185, miR-365, and miR-503. Further, miR-146a was related to progression of atherosclerosis by decreasing cell migration and promoting macrophage entrapment in the vessel wall [
111].
Elevated expression of miR-192, miR-194 and miR-34a in serum EVs was observed in HF patients after acute myocardial infarction [
106]. Serum exosomal miR-92b-5p was increased in patients with HF due to dilated cardiomyopathy ant this miRNA was suggested as biomarker for diagnosis of HF [
107].
EV miRNAs were also related to cardiovascular risk factors (i.e., diabetes, dyslipidemia, obesity, MetS). Cardiomyocytes isolated from type 2 diabetic rats had inhibitory effects on myocardial angiogenesis through the EV transfer of miR-320 into endothelial cells [
136]. miR-24 and miR-130a were downregulated in plasma EVs of patients with familial hypercholesterolemia (FH), and miR-130a levels were inversely related to coronary atherosclerosis in suspected CAD patients, suggesting their role as potential biomarkers of FH and CAD [
73].
A recent study using abdominal adipose tissue-derived mesenchymal stem/stromal cells showed four downregulated miRNAs (miR-136, miR-4798, miR-12,136, miR-222) and nine upregulated miRNAs (miR-630, miR-144, miR-143, miR-4787, miR-769, miR-8074, and miR-181a) from EVs of MetS patients. These deregulated miRNAs might control genes, which were associated with cellular senescence, cell cycle, metabolic processes, and apoptosis pathways [
137].
3.2. Extracellular Vesicles Carrying Proteins
Differences in EV protein levels occur in response to a variety of physiological or pathological stimuli. The protein profile might change already in a very early stage of the disease, which makes this content a potential early biomarker [
115]. The EV protein cargo is heterogenous and dependent on the cell type or biofluid of origin [
138].
EV proteins were suggested to be prognostic biomarkers of cardiovascular events. In this context, a prospective study demonstrated the increase of circulating CD31/Annexin 5-positive EVs as an independent predictor of cardiovascular risk in patients with stable CAD. High levels of CD31/Annexin 5 EVs were associated with higher incidence of death caused by CVD and higher need for revascularization [
112,
139].
A study of EV proteome of patients with myocardial infarction identified six novel EV protein markers of myocardial damage related to three pathways: complement activation (C1Q1A and C5), platelet activation (GP1BA and PPBP), and lipid metabolism (APOD and APOC3) [
113]. Increased plasma levels of CD144-EVs were also suggested to be predictive of cardiovascular complications (i.e., ACS, ischemic stroke, revascularization, and death) in patients with high risk for CAD [
114,
139].
The link between EV proteins and atherosclerosis was described in a study, which showed that hypercholesterolemic patients with subclinical lipid-rich atherosclerotic plaques have a higher abundance of CD45/CD3-derived EVs than those in patients with fibrous plaques [
100].
EV protein levels showed an association with stress-induced ischemia, especially proteins known to be related to inflammatory cascades such as SerpinC1, SerpinG1, CD14, and Cystatin C [
115]. Serum EV proteins, such as cystatin C, polygenic immunoglobulin receptor (pIgR) and complement factor C5a (C5a), were suggested to be associated with ACS [
117,
140]. mCRP carried by monocyte-derived EVs was associated with inflammatory process in patients with CAD [
52]. EVs can transport and delivery pro-inflammatory mCRP in endothelial cells [
118]. mCRP carried by endothelial cell-derived EVs was also increased in patients with peripheral artery disease, and it was suggested to be a pro-inflammatory molecule and a potential indicator of vascular disease risk [
119].
Plasma levels of EVs enriched in cystatin C, CD14, serpinG1, and serpinF2 were markedly increased in HF patients. These EVs proteins, previously related to systemic vascular events, were associated with high risk of HF in patients suspected of acute HF [
116].
A recent study, using human cardiovascular cells, demonstrated that Annexin A1 induces EVs aggregation and microcalcification formation that promote CVD. These findings could lead to development of therapeutic strategies in CVD [
120].
Urinary levels of EV proteins were decreased in patients with unstable CAD, however levels of CD45+ and CD11b+ EVs were increased and CD16+ EVs were decreased. These urinary EV proteins were suggested to be associated with CAD progression [
108]. High levels of urinary EVs enriched in nephrin and podocalyxin were observed in patients with hypertension and these EV proteins were proposed to be useful diagnostic biomarkers [
121]. Urinary EVs released by senescent nephron cells had increased levels of p16 (senescence marker) in patients with hypertension as compared to healthy volunteers. Urinary p16-positive EVs could serve as an early marker of nephron senescence and could be useful in disease management and therapeutic follow-up [
122].
3.3. Extracellular Vesicles Carrying Lipids and Metabolites
Lipids are important components of vesicle bilayer membranes and specifics lipids, such as cholesterol and sphingomyelin, are enriched in vesicles compared to their parental cells and it might modulate recipient cell homeostasis [
18,
141]. Then, lipids are emerging as very important players for the physiological functions of these vesicles [
142]. The first studies relating to the lipid composition of EVs were performed on prostate-derived EVs found in seminal fluid about twenty years ago [
142,
143]. The data have been included in the EVs databases such as Vesiclepedia [
144], EVpedia [
145], and Exocarta [
146].
EVs lipids interact with receptors on the target cell and are thereafter internalized intro endosomes where they concentrate the bioactive lipids that they carry modulating endogenous cell lipid metabolism [
18]. Since lipids are essential structural and functional constituents of EVs [
142], the use of EV lipids as biomarkers of CVD may be promising, however, there are only a few studies on this topic.
EVs can carry ceramides, sphingomyelin, lysophosphatidylcholine, arachidonic acid, and other fatty acids, cholesterol, prostaglandins, leukotrienes, and active lipolytic enzymes (such as phospholipase A2) on their membrane or within their lumen, and their lipid composition can be modified by in vitro manipulation [
18]. Circulating EVs were enriched with different sphingolipid species (ceramides, dihydroceramides, and sphingomyelins) in patients with STEMI, and lipid levels correlated with cardiac troponin, leucocyte count, and lower left ventricular ejection fraction [
123].
The amount of lipids in the shed EVs could be directly related to atherosclerosis, once accumulation of these lipids was associated with foam cell formation and apoptosis in macrophages mediated by toll-like receptors, which can lead to atherosclerosis [
8,
147]. EVs can be released by activated platelets, which are rich in phosphatidylserine, contributing in thrombin generation and promoting thrombosis [
38,
148]. Activated platelets also release EVs rich in arachidonic acid, which contributes to thrombosis in the recipient cells by the promotion of the cell adhesion and stimulation of prostacyclin and thromboxane A2 synthesis [
149,
150].
A pioneering study showed that urinary EV metabolites (4-aminohippuric acid, citric acid, and N-1-methylnicotinamide) were altered in patients with high cardiovascular risk. Urinary EV levels of 4-aminohippuric acid were increased, whereas citric acid and N-1-methylnicotinamide were reduced in patients with high cardiovascular risk, suggesting an important role of EV metabolites as biomarkers of CVD [
124].
This entry is adapted from the peer-reviewed paper 10.3390/biom11030388